What is the electron configuration of F^-?
1 Answer
Explanation:
A good starting point for when you must find the electron configuration of an ion is the electron configuration of the neutral atom.
In your case, you must find the electron configuration of the fluoride anion,
Fluorine is located in period 2, group 17 of the periodic table and has an atomic number of
Its electron configuration will be
"F: " 1s^2 2s^2 2p^5
Now, the
Notice that the 2p-subshell of the neutral atom contains
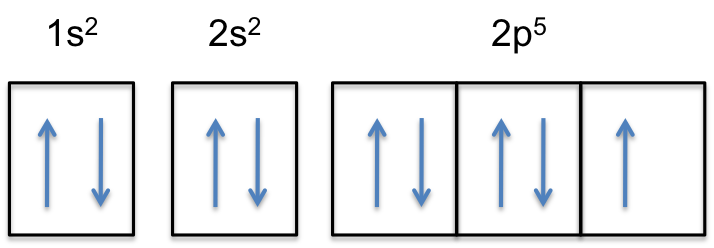
This means that the
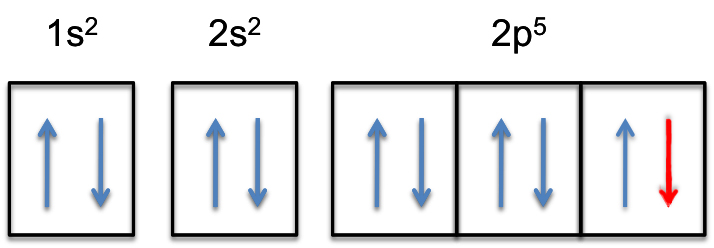
The 2p-subshell will now be completely filled, i.e. it will hold
The electron configuration of the fluoride anion will thus be
color(green)(|bar(ul(color(white)(a/a)color(black)("F"^(-):color(white)(a) 1s^2color(white)(a) 2s^2 color(white)(a)2p^6)color(white)(a/a)|)))
Notice that the fluoride anion has a total of
Because the fluoride anion is isoelectronic with neon,
"F"^(-): ["Ne"]
Here

