Besides vanillin, other fragrant aromatic compounds include anisaldehyde, cinnamaldehyde, and coumarin. How would you predict where bromination would take place in these structures?
1 Answer
Jun 24, 2016
Here's how I would argue.
Explanation:
Anisaldehyde
The structure of anisaldehyde is

The aldehyde group directs substituents to the meta positions (
The methoxy group also directs substituents to the ortho positions (
∴ The bromination product is 3-bromo-4-methoxybenzaldehyde.
Cinnamaldehyde
The structure of cinnamaldehyde is

The
Hence, we will get addition across the external bond.
The bromination product is 2,3-dibromo-3-phenylpropanal.

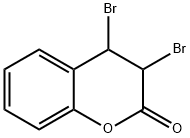
Coumarin
The structure of coumarin is

The alkene double bond is more reactive than those in the benzene ring.
∴ We will get addition of bromine across the double bond.
The product will be 3,4-dibromocoumarin.