What is the difference between tautomerism and metamerism?
1 Answer
Tautomerism and metamerism are different types of structural isomerism.
Explanation:
Structural isomerism is a form of isomerism in which molecules have the same molecular formula but their atoms are connected in different orders.
Structural isomerism is divided into:
• Chain isomerism
• Positional isomerism
• Functional group isomerism
• Tautomerism
• Metamerism
Tautomerism
Tautomerism is a dynamic equilibrium between two compounds with same molecular formula.
The most common form of tautomerism is keto-enol tautomerism.
A carbonyl compound containing at least one α-hydrogen atom is converted to an enol by the transfer of an α-hydrogen onto the oxygen atom. For example,
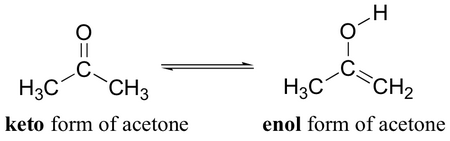
(from chemwiki.ucdavis.edu)
Metamerism
Metamerism is a type of structural isomerism in which different alkyl groups are attached to the same functional group.
For example, diethyl ether and methyl propyl ethers are metamers.
They both contain the ether functional group, but they have different alkyl groups attached to the oxygen atom.

