How do functional groups work?
1 Answer
It alters the property of the organic compound (assuming alkane is the main reference)
Explanation:
An alkane is usually unreactive (with the exception of reaction with strong acids or high T)). i.e. They cannot form hydrogen bond, ionic ion-dipole bond, ionic bond.
They also rarely release/donate proton (H+) nor receive electron.
And neither are they attracted to molecules that are electron "deficient" (an electrophile) or to molecules that possess "excess" electron pair (nucleophile).
How do functional groups alter these behaviour/properties?
Formation of hydrogen bonding can take place if the organic molecule contains hydroxyl group (OH). This helps alter properties like increased solubility inside water. Take ethanol for example. the hydrogen bonding increased.
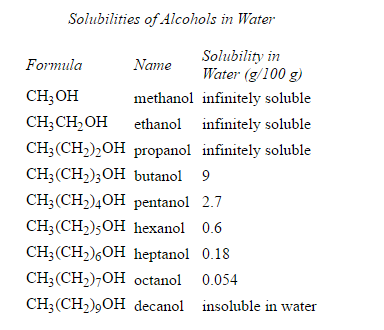
cite: http://chemed.chem.purdue.edu/genchem/topicreview/bp/2organic/alcohols.html#hydro

cite: http://academic.pgcc.edu/~ssinex/struc_bond/solubilities.htm
The presence of functional group like carboxylic acid helps release proton (H+) which allows it to react with basic molecules. Through this, it can produce ester bonding through reaction with alcohol (alcohol can be acidic or basic).
Meanwhile, alkenes possess pi-bonds. These electrons attracts electrophile (electron-loving) molecules like halogen (like bromine)
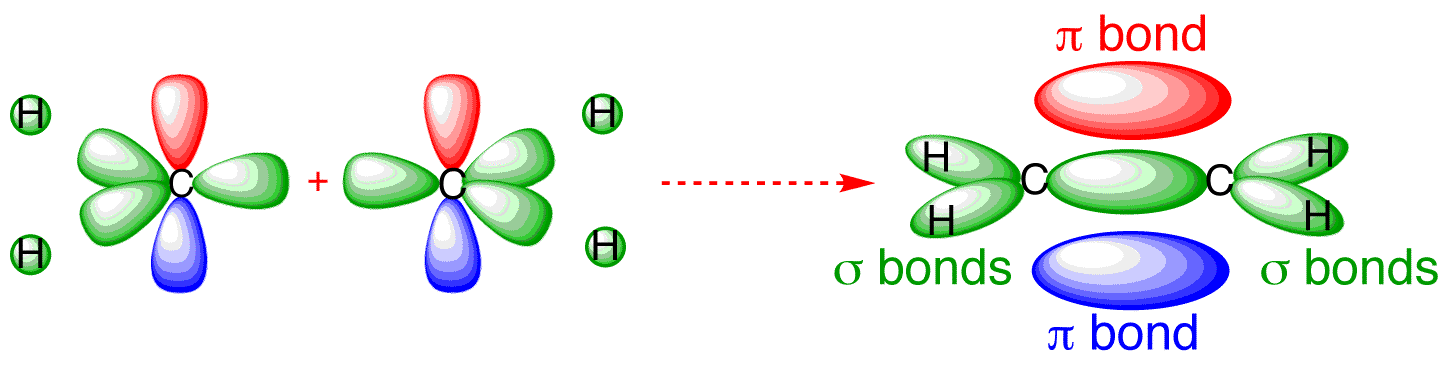
cite: http://www.chemtube3d.com/orbitalsethene.htm
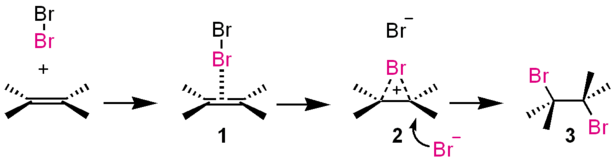
cite; https://en.wikipedia.org/wiki/Electrophile

