As atomic orbitals form they require how many electrons to fill the orbital?
1 Answer
I think you're confusing atomic orbitals with molecular orbitals.
When molecular orbitals form, two valence electrons are required to be situated in between two atoms for a molecular orbital, forming a chemical bond.
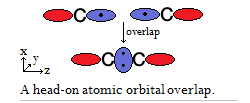
So for this hypothetical
Here's the thing - atomic orbitals don't actually require any electrons in them.
Case in point:
When atomic orbitals overlap, they will form molecular orbitals (bonding and antibonding, such that the number of molecular orbitals is equal to the number of atomic orbitals). That is...
The electron population within molecular orbitals constitute the chemical bonds made between atoms!
- Any sigma (
#sigma# ) bond requires the head-on overlap of atomic orbitals. - Any pi (
#pi# ) bond requires the sidelong overlap of atomic orbitals.
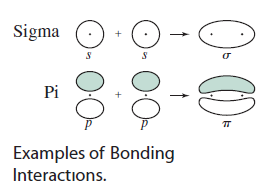
(The dots in the diagram represent atoms, so those are not electrons.)
Actually, each
- For
#sigma# bonding molecular orbitals, the electrons are right smack dab in between the atoms. - For
#pi# bonding molecular orbitals, the electrons are in between the atoms, but in the lobe above the atoms.
(How the electrons are shared or transferred is another story, but what matters is that there are two.)

