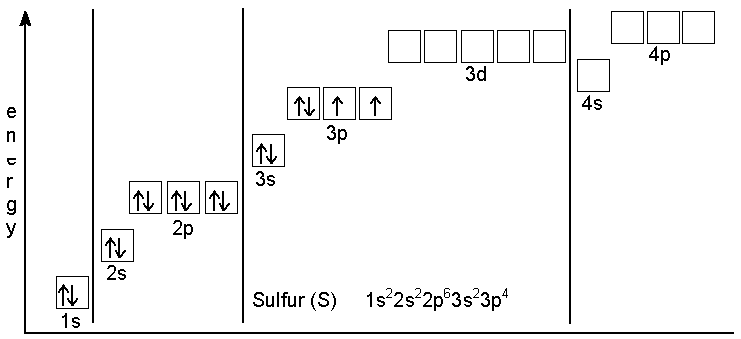
What element has the electron configuration of 3s^2 3p^43s23p4 in its outermost shell?
1 Answer
Sulfur.
Explanation:
The first thing to note here is that for a given element, the energy level on which the outermost electrons reside gives you the period in which the element is located in the periodic table.
In this case, your element is said to have the electron configuration
color(red)(3)s^2 color(red)(3)p^43s23p4
in its outermost shell, which implies that this element is located in period
Now, the great thing about elements located in period 3 is that they are all main-group elements, i.e. they are located in group 1, 2, or 13 through 18.
Consequently, the total number of electrons located in the outermost shell gives you the group in which the element is located in the periodic table.
In this case, the outermost shell contains
"2 e"^(-) ->2 e−→ located in the 3s orbital
"4 e"^(-) ->4 e−→ located in the 3p orbitals
The total number of electrons located in this shell is
"2 e"^(-) + "4 e"^(-) = color(blue)("6 e"^(-))2 e−+4 e−=6 e−
which means that the element is located in group
"S: " 1s^2 2s^2 2p^6 3s^2 3p^4S: 1s22s22p63s23p4