Why is O2 paramagnetic?
1 Answer
Sep 25, 2016
Explanation:
The Lewis structure of

It shows that all the electrons in oxygen are paired, so oxygen should be diamagnetic.
Yet oxygen is paramagnetic.
The correct explanation comes from Molecular Orbital theory.
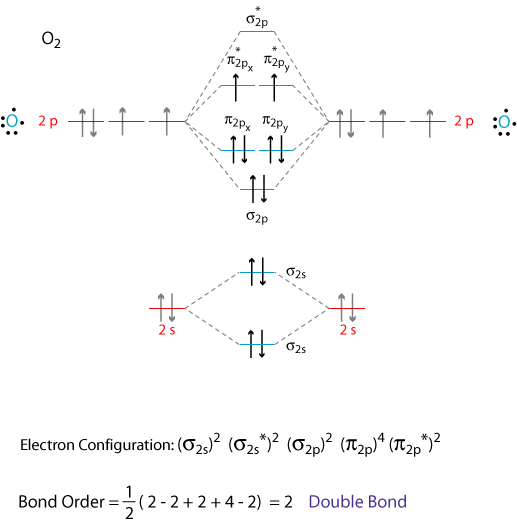
The atomic orbitals of the
We add the 12 valence electrons according to the Aufbau principle.
The last two electrons go into separate, degenerate π orbitals, according to Hund's Rule.
Thus, oxygen has two unpaired electrons and is paramagnetic.

