Why does #["Co"("NN"_3)_6]^(3+)# form an inner orbital complex but #["CoF"_6]^(3-)# form an outer orbital complex?
1 Answer
I am assuming you mean
Explanation:
One characteristic of the transition elements is their ability to form complex ions. These consist of a central metal ion surrounded by electron - donating species called ligands.
Cobalt(III) ions have the electron configuration:
Fig 1 (a)
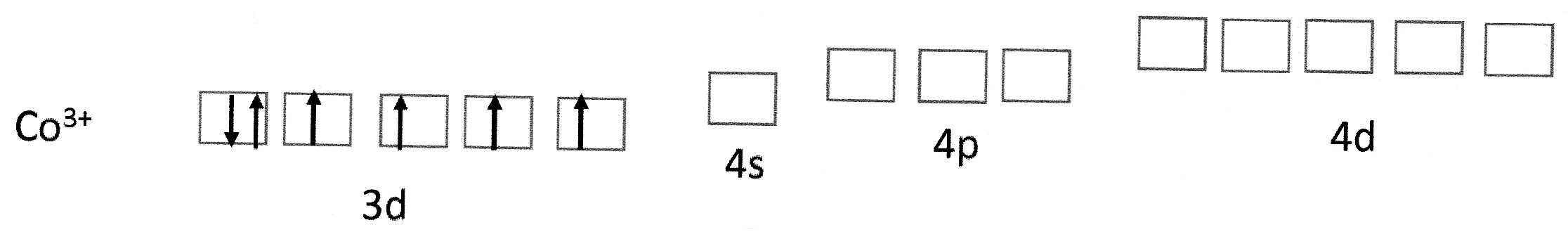
The empty 4s, 4p and 4d orbitals are quite close in energy to the 3d orbitals.
This means that ligands with available lone pairs such as ammonia molecules are able to donate electrons into these empty orbitals and form co - ordinate bonds.
Quite a common stable arrangement is with 6 ligands.
An example is hydrated
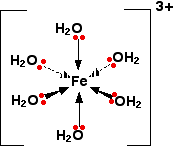
The d orbitals look like this:
Fig 1 (b)

Because of the octahedral symmetry of the complex the
The other 3d orbitals have lobes which project between the ligands and are relatively more stable. This is shown here:
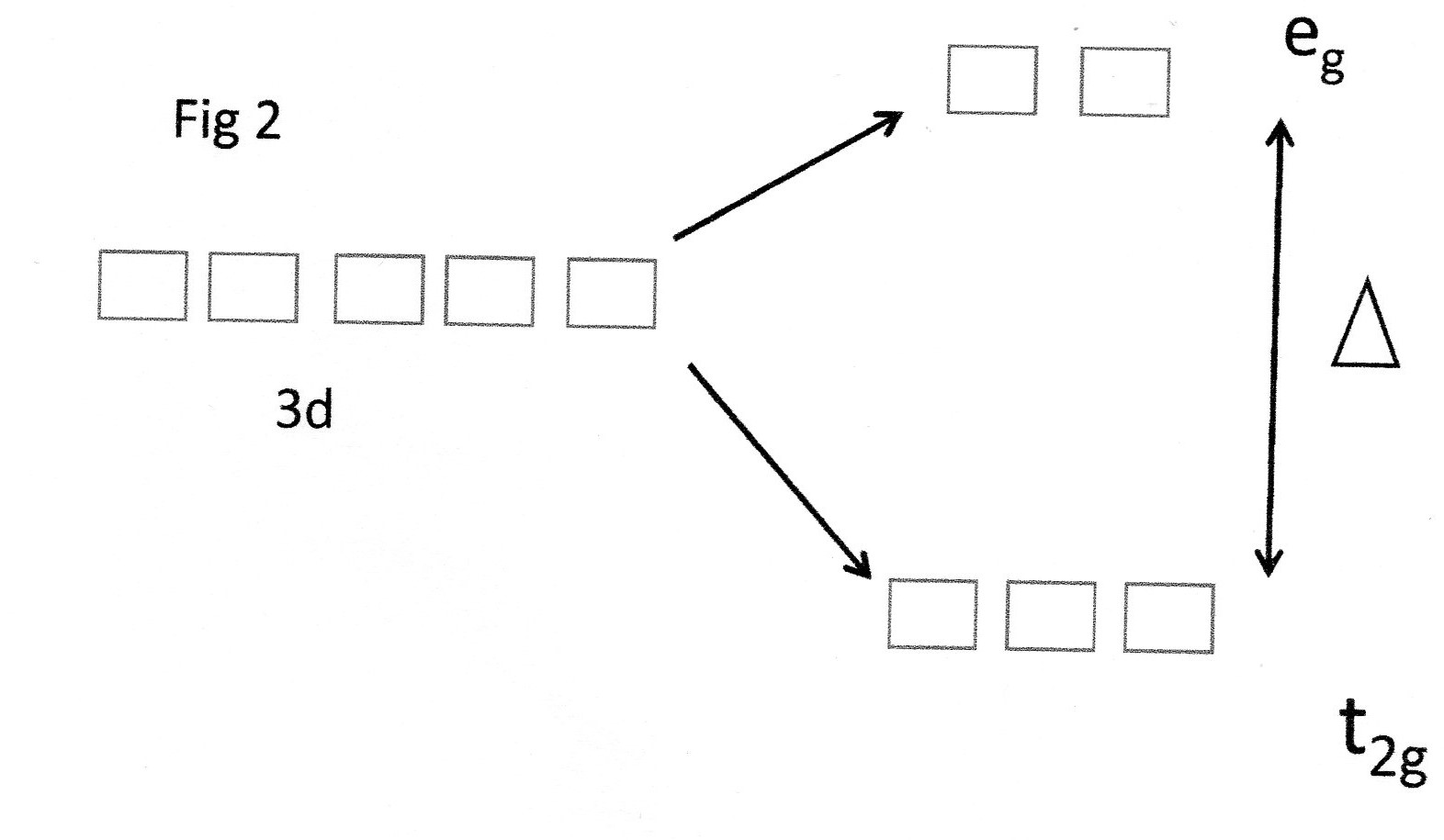
These orbitals are given the symmetry terms
The important point to note here is that the value of
The spectrochemical series lists the ligands in order of
Some are listed here:
I- < Br- < S2- < SCN- < Cl- < NO3- < F- < OH- < C2O42- < H2O < NCS- < CH3CN < NH3 < en < bipy < phen < NO2- < PPh3 < CN- < CO
This tells us that
For the ammonia complex the value of
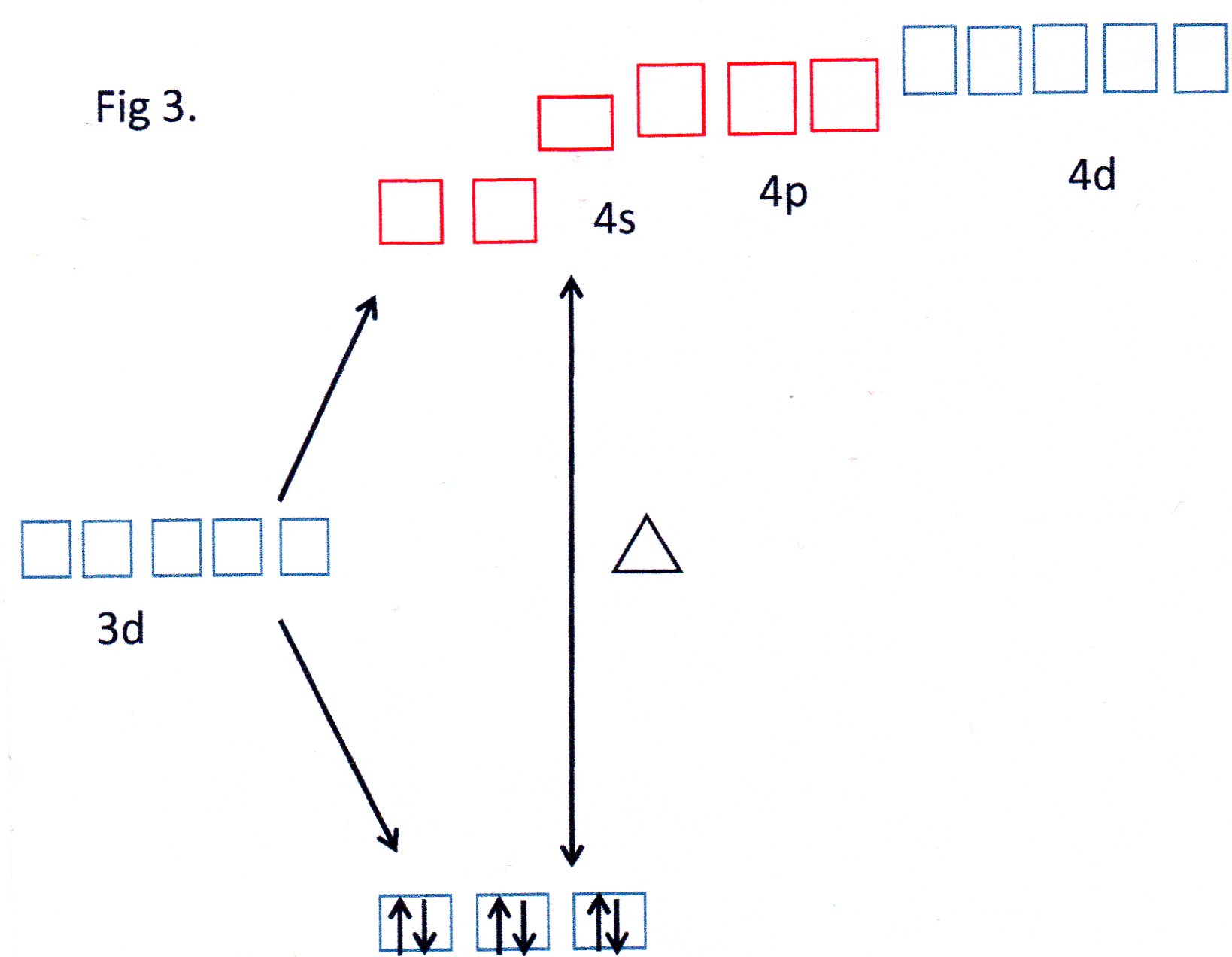
This is termed a "low - spin" complex.
You can see from Fig 3 that the orbitals shown in red are able to each accept a pair of electrons from each
The 3d, 4s and 4p orbitals effectively reorganise themselves to form 6 equivalent orbitals which are described, therefore, as
Because inner 3d orbitals are used this can be referred to as an inner orbital complex .
In the case of the
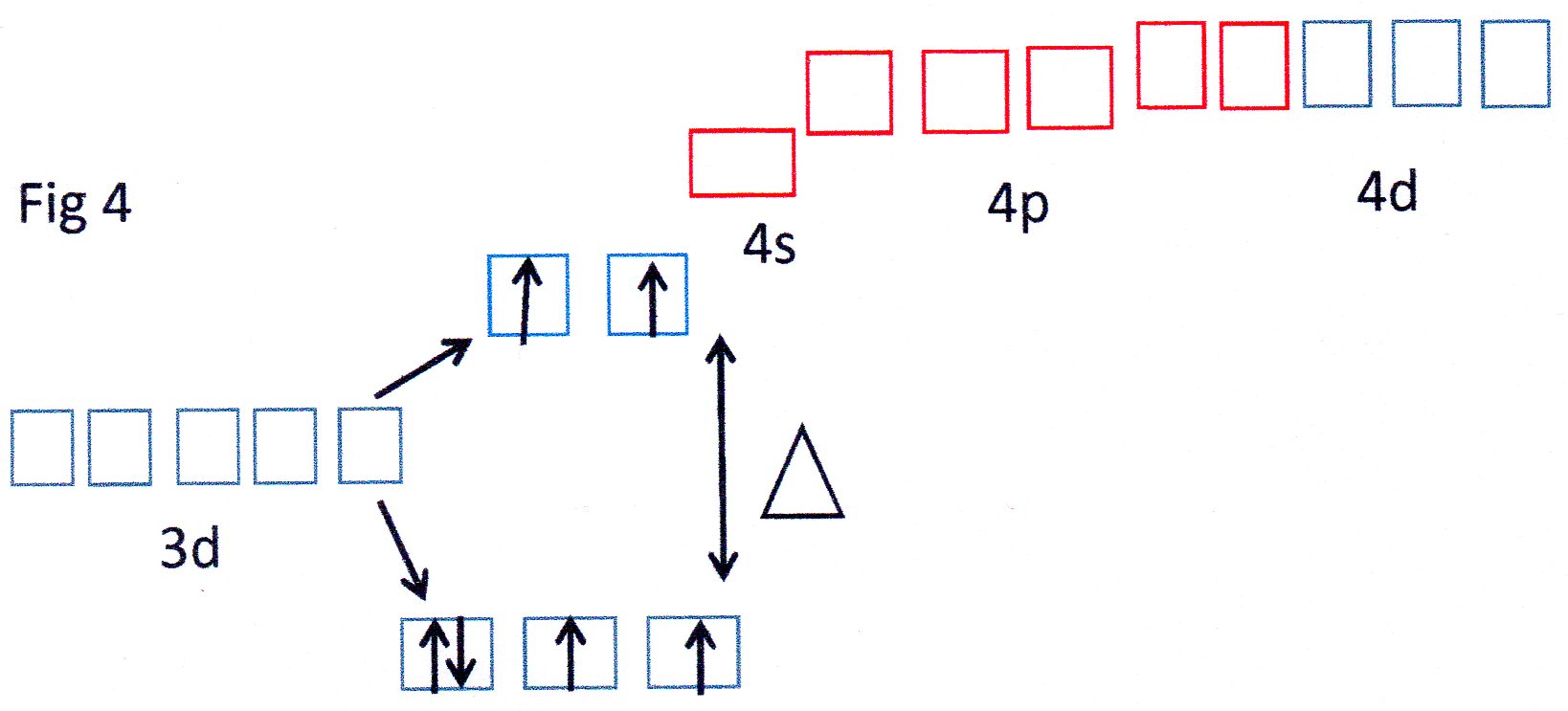
Because the
Because the outer 4d orbitals are now being used this can be described as an outer orbital complex.

