How does the ionic radius of a nonmetal compare with its atomic radius?
2 Answers
The ionic radius of a non metal is greater than the atomic radius of the neutral atom
Explanation:
When a non metal gains electrons to become a negative ion the radius increases. The number of protons remains the same so the pull on the electrons is the same. However as the number of electrons increases with the extra electrons the average pull on the electrons is less. The decreased pull causes the distance from the nucleus to increase.
The ionic radius of a nonmetal is greater than its atomic radius.
Explanation:
When you add an electron to a nonmetal, you are changing two properties:
1. The added electron is slightly more shielded by the electrons already there.
For example, the effective nuclear charge (
For a
This decreased attraction should cause an increase in the ionic radius.
2. The added electron increases electron repulsions.
These should also increase the ionic radius.
Which is the bigger effect?
The atomic radius of
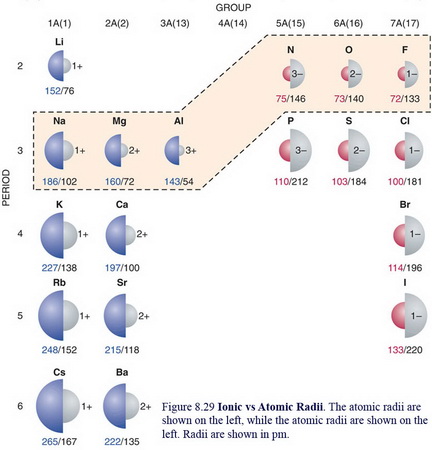
(From www.chem.uwec.edu)
The near-doubling of the radius cannot be explained by the relatively small increase in nuclear shielding.
Rather, the increased size results from the increased electron repulsions caused by the added electron.

