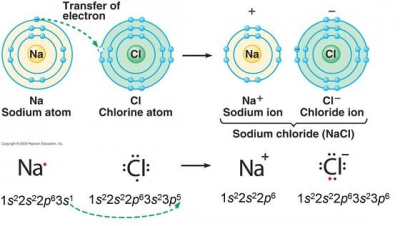
What is a possible ion that has this electron configuration: #1s^2, 2s^2, 2p^6, 3s^2, 3p^6#?
1 Answer
Nov 6, 2016
It would be the chloride ion, with a charge of
Explanation:
A neutral chlorine atom has the electron configuration
The image below represents the formation of the ionic compound sodium chloride,