What is the difference between Keesom interactions and, London dispersion forces?
1 Answer
Dec 2, 2016
Keesom interactions are dipole-dipole interactions, while London dispersion forces are induced dipole-induced dipole interactions.
Explanation:
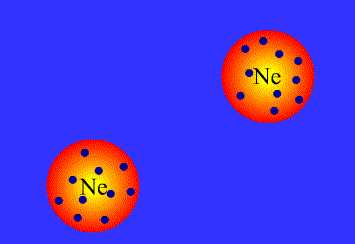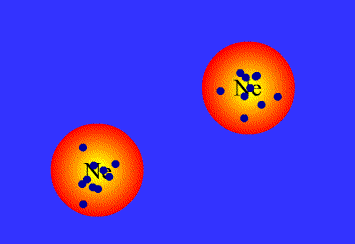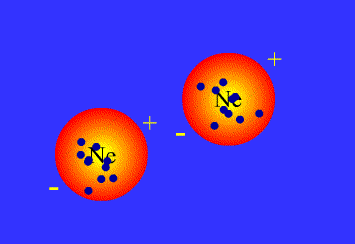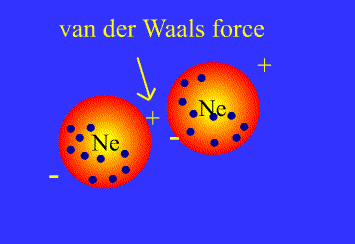
Both types of interaction contribute to van der Waals forces.
Keesom interactions
Keesom interactions are the electrostatic interactions among the permanent dipoles of polar molecules.
They arise when the
London dispersion interactions
London dispersion interactions arise when a temporary imbalance of electrons of a nonpolar molecule induce a temporary dipole in another nearby molecule.
You can find more details about the differences in this Socratic answer.


