What type of bonds are being broken when you melt ice?
1 Answer
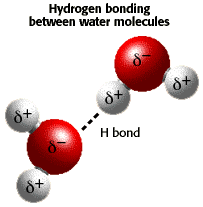
Hydrogen bonds. Hydrogen bonds are relatively weak bonds, and don't involve the sharing or transfer of electrons. The bond between each oxygen and hydrogen atom is a polar covalent bond, which involves the unequal sharing of electrons.
Explanation:
Water molecules are polar. The oxygen atom has a partial negative charge
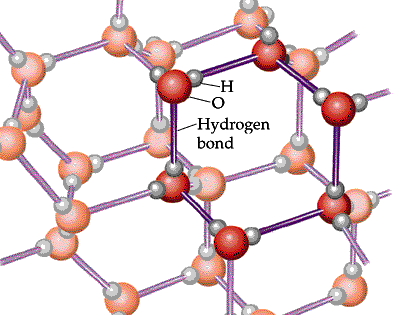
The following diagram shows the hydrogen bonds that at its freezing point cause the water molecules to form a crystalline structure, increasing volume, and decreasing density. Water is at its lowest density at