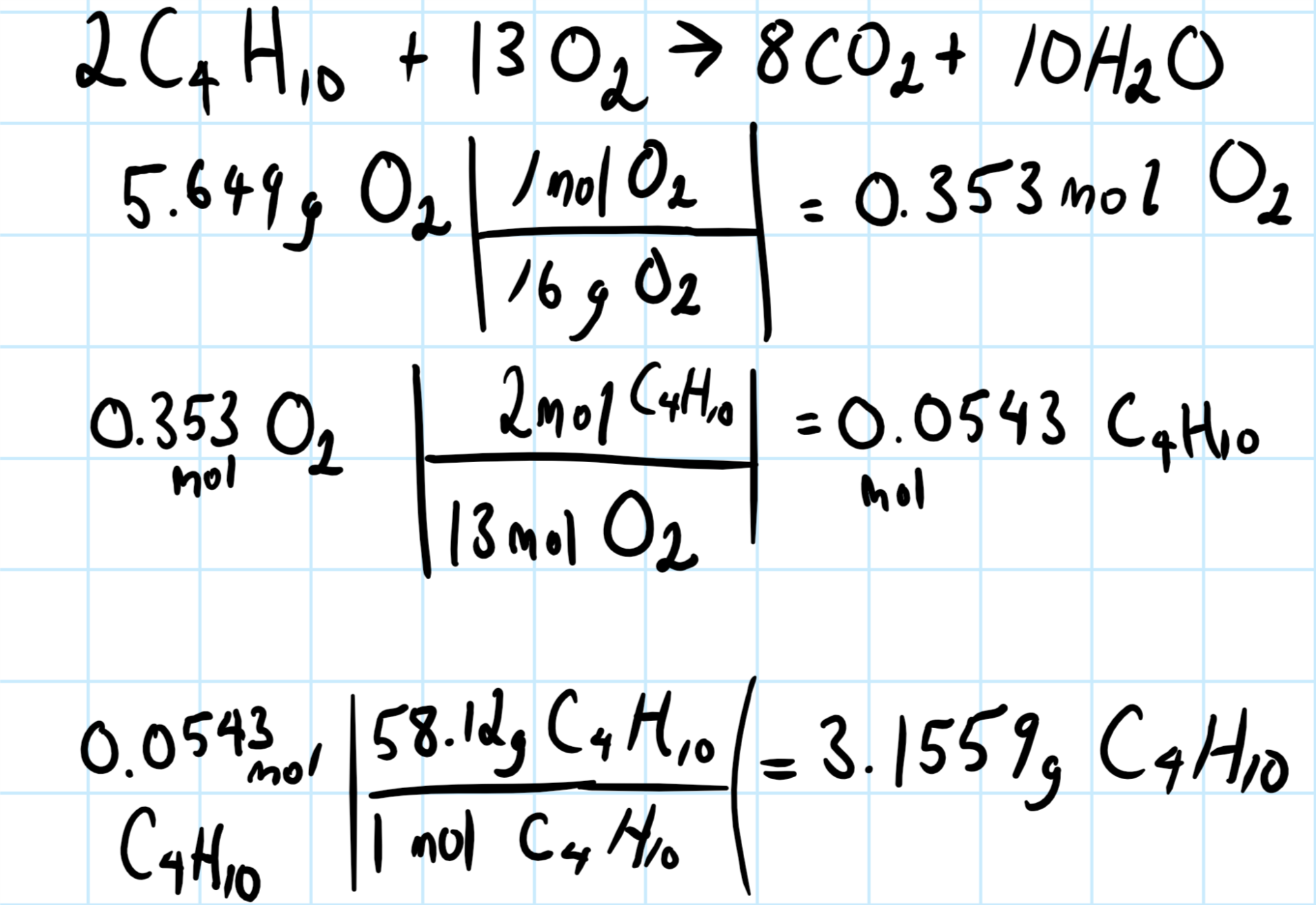Question #fea87
1 Answer
Dec 29, 2016
3.156 g
Explanation:
I'm going to assume the balanced chemical system is:
You then need to convert the given relevant quantity to moles, and use the molar ratios to find the gram butane required to react with the given quantity about the chemical system.
Consider the graphic below, showing the balanced chemical equation for the system, and the calculations: