The atom having the valence-shell configuration #4s^2 4p^5# would be in what group and period?
1 Answer
Jan 4, 2017
The atom would be in Period 4 and Group 17.
Explanation:
The valence shell configuration is
The principal quantum number (4) gives you the Period number.
Start with H in Period 1 and count down to the fourth row (Period 4).
That brings you to
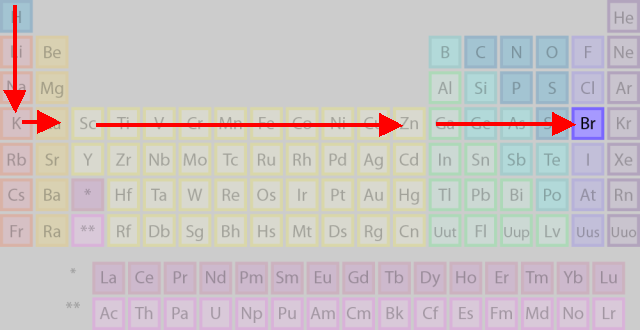
(Adapted from Chemistry - About.com)
Now, start counting to the right through the
Now that you have come to the
This brings you to the desired atom,
You have counted horizontally through 17 atoms, so

