What is the bond formed when atoms share electrons?
1 Answer
Jan 6, 2017
A covalent bond forms when atoms share electrons.
Explanation:
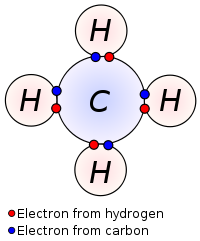
When two atoms form a covalent bond, they share their valence electrons in order to achieve an octet (8 valence electrons), except for hydrogen which bonds to achieve a duet (2 valence electrons). Covalent bonding makes the atoms stable. Most covalent bonds form between nonmetallic atoms.
The diagram below shows a methane molecule