Why are aromatic compounds stable?
1 Answer
They have a great deal of resonance stabilization, which is due to delocalization (the spreading out) of the
In general, the more spread out electron density is, the less interelectronic repulsion there is, and the less electrons are "stored" on average within a particular orbital, which decreases the overall energy of the molecule. Sometimes the resonance helps a little, sometimes a lot...
For instance, consider the hypothetical 1,3,5-cyclohexatriene, and compare its enthalpy of complete hydrogenation to that of benzene:
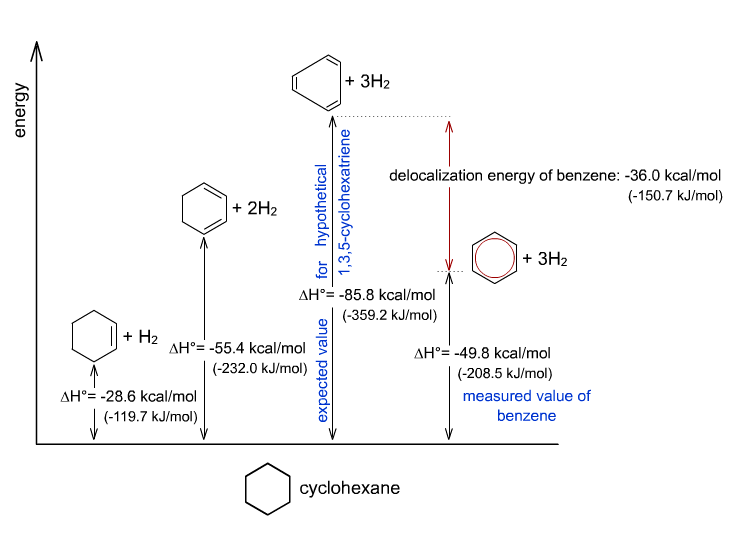
(This would indicate how much energy input it requires to break all the
In theory, if benzene were just like cyclohexatriene (i.e. no resonance at all), its double bonds would absorb
In reality, however, benzene has about

