How would I get the oxidation numbers and balance this redox reaction? #"H"_2"O"_2 + 2"C""H"_3"-""S""-""H" → 2"H"_2"O" + "CH"_3"-S-S-CH"_3#
1 Answer
WARNING! Long answer! Here's how I do it.
Explanation:
You get the oxidation numbers from the Lewis structures.
The rules for counting the valence electrons are:
- Electrons shared between identical atoms are shared equally.
- Electrons shared between nonidentical atoms belong entirely to the more electronegative atom.
- Lone pairs belong entirely to the atom that has them.
You count the electrons using these rules. Let's call this number E.
Then you subtract from the number of valence electrons in an isolated atom V.
The formal charge
The Lewis structure is
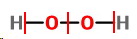
I have marked the allocation of electrons by vertical red lines.
Each
For H,
For O,
The structure is
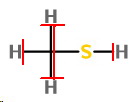
Here the formal charges are
For H,
For C,
For S,
You can do this one.
The formal charges are
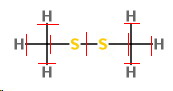
The formal charges are
For
For
For
Now, we put these oxidation numbers above the atoms in the equation.
The changes in oxidation number are


