How does dipole moment affect molecules in solution?
1 Answer
Dipole moments result in electric interactions between solute and solvent that are essential for the two chemicals to mix adequately for a solution to form.
Explanation:
If a solution is to form when two chemicals are mixed, three processes must occur:
- particles in the solute must be separated
- particles in the solvent must be separated
- the particles of solvent and solute must interact
The first two of these involve pulling molecules or ions apart that are strongly interacting (otherwise they would not be solids or liquids). This is why a strong interaction is needed between solute and solvent - to "wrestle" the solute particles apart, and keep them apart, once the mixing has occurred. The interaction between ions in the solute and polar solvent molecules is shown in the diagram below
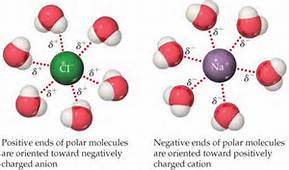
This is called hydration if the solvent is water.
A similar process can occur if both solvent and solute are polar, only in this case, the interaction is between two dipoles.


