Question #60897
1 Answer
The energies are almost equal, but slightly different, because the electrons in some orbitals spend more time closer to the nucleus.
Explanation:
In atoms of elements with more than one electron, each principal energy level is split into different sublevels.
For example, the order of increasing energy for orbitals in the fourth level is
 upload.wikimedia.org
upload.wikimedia.org
This is because the
We can see this if we compare the "radial distribution functions" for each type of orbital, that is, the probability of finding an electron at a given distance from the nucleus.
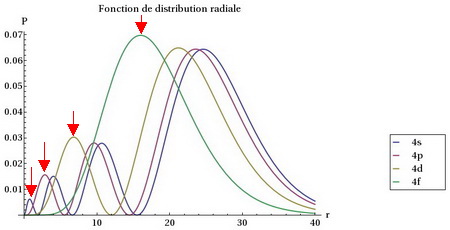 Radial distributions
Radial distributions
(Adapted from www.f-legrand.fr)
We see that the
The
Thus, a
Next comes the

