What is the electron configuration of an element with atomic number 12?
2 Answers
Explanation:
The element with the atomic number 12 is magnesium. The atomic number indicates the number of positively charged protons in each magnesium nucleus. A neutral atom has equal numbers of protons as negatively charged electrons. The electron configuration of a neutral magnesium atom is:
The Aufbau diagram can help you determine the correct order in which the electrons will fill the orbitals in an atom.
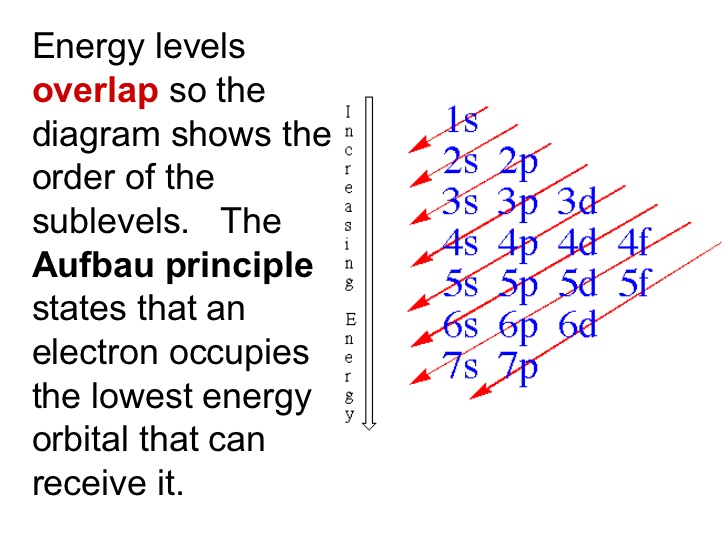
Explanation:
The element with atomic number 12 is Magnesium. It has 12 electrons:
2 electrons in the 1st orbital in an s-shell
8 electrons in the 2nd orbital: 2 in an s-shell and 6 in the p-shell
and 2 in the 3rd orbital, an s-shell.
You can use the Aufbau principle to help with the order.


