In the chemical equation, how do the number of atoms of each element in the reactants compare to the number of atoms of each element in the products?
1 Answer
Feb 19, 2017
Atoms of the reactant(s) must equal the atoms of the product(s).
Explanation:
According to the Law of Conservation, all atoms of the reactant(s) must equal the atoms of the product(s).
As a result, we need to balance chemical equations. We do this by adding in coefficients to the reactants and/or products. The compound(s) itself/themselves DOES NOT CHANGE.
Unbalanced chemical equations (AKA skeleton equations) disobey the law and should never be used in any chemical calculation, like stoichiometry.
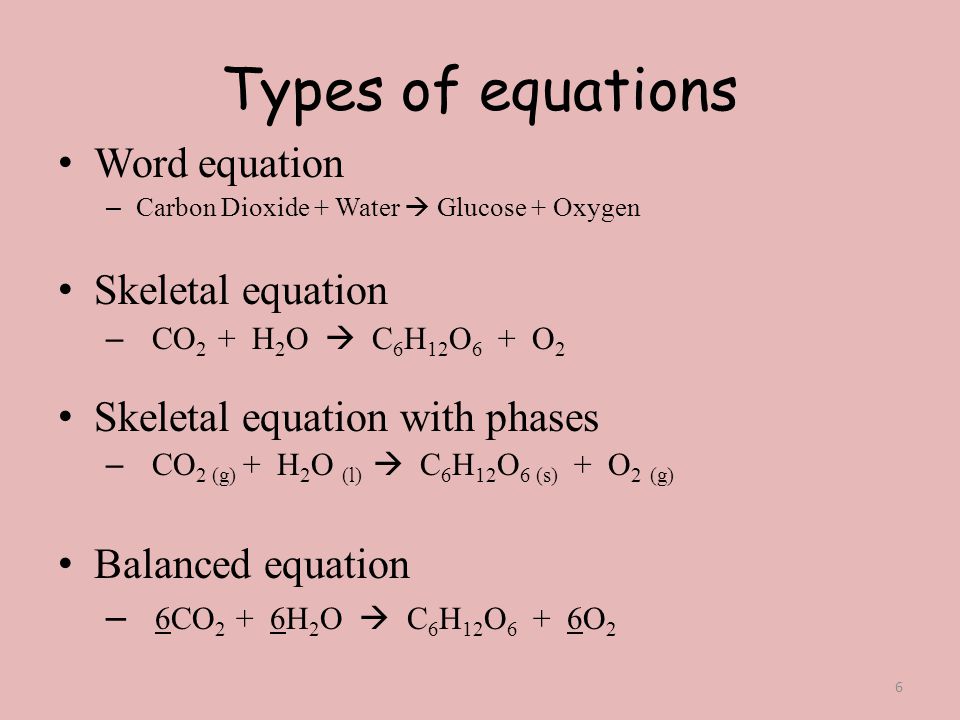
Here we see the balanced equation has equal numbers of atoms on each side of the yield sign:
- 18 Oxygen atoms,
- 12 Hydrogen atoms,
- 6 Carbon atoms
Hope this helps :)

