Question #678ec
1 Answer
Mar 2, 2017
Element number 13 has 1 unpaired electron.
Explanation:
Since
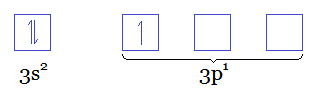
That means that at the last level (the
Element number 13 has 1 unpaired electron.
Since

That means that at the last level (the