How do you draw the electron configuration for oxygen, using Hund's Rule and the Pauli Exclusion principle to do it?
1 Answer
Fill all of the lower ‘shells’ first, then using the stated ‘rules’ put the valence electrons in their proper electron shells.
Explanation:
Hund’s rule is the most important, as it tells us that we can’t “double up” any electrons in orbitals until all of them in a particular shell have at least one. The Pauli Exclusion Principle really just says that electrons can’t be superimposed on each other, but it does not affect the filling of the orbital shells.
For oxygen, with an atomic number of 8, has 8 electrons. The electron configuration is thus
If a graphical depiction is desired instead of the “notation” one already given, it is usually a series of lines or boxes representing the orbitals with ‘up’ and ‘down’ arrows placed in them to represent the electrons in an orbital and their spins. For oxygen it would look like this:
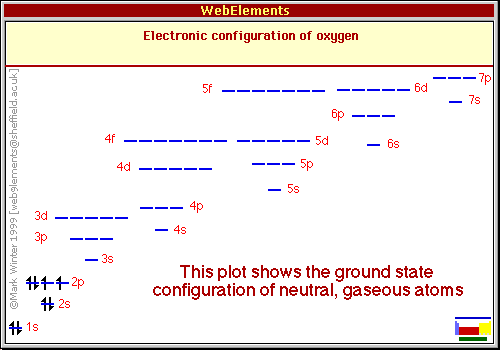
https://www.webelements.com/oxygen/atoms.html (copyright info embedded in graphic)

