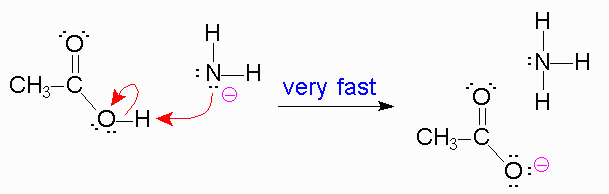Amides are molecules that can form from a carboxylic acid and an amine group. In our body, when two amino acids come together to react, they form an amide bond, releasing #"H"_2"O"# in the process. This type of bond is also called a peptide bond.
#color(white)(aaaaaaaaaaaaaaaaaa)#
#color(white)(aaaaaaaaaaaaaaaaaa)# Mechanism
#color(purple)(1.)#
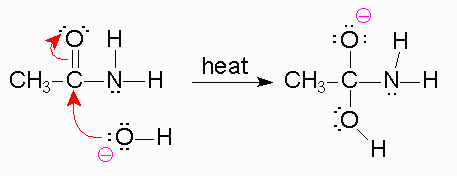
To begin, we know that in the first step, a strong base is going to be used like #"NaOH"#, where the #color(blue)("OH"^(-)# will act as the nucleophile and attack the electrophile, the partial positive carbon of the carbonyl group.
Now, when the bond forms between the #"oxygen"# of the #"OH"^-# group and the carbon, the #pi# #"bond"# of the carbonyl is broken and the electrons are pushed toward oxygen, leaving it with a negative #-1# charge.
#color(white)(---------------------)/color(white)(a)#
#color(purple)(2.)#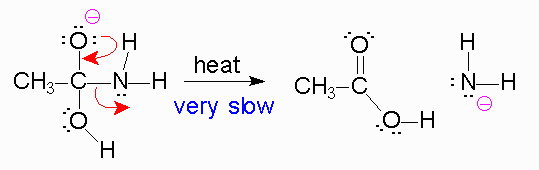
#color(blue)("Oxygen will try to remove this negative charge by reforming")# #color(blue)("a double bond with the carbon")#. But since we know that the #"carbon"# atom cannot form more than 4 bonds, the bond between it and the amine's #"nitrogen"# has to be broken. #color(blue)("The electrons will be pushed onto the nitrogen of the amine and the")# #color(blue)("amine will act as the leaving group (a poor one at that)")#
*The reason the amine is a poor leaving group is because the amine is a strong base, and strong bases are very unstable. Leaving groups are based off their stability in solution, like the halides, tosylate, #"H"_2"O"#
#color(white)(---------------------)/color(white)(a)#
#color(purple)(3.)#
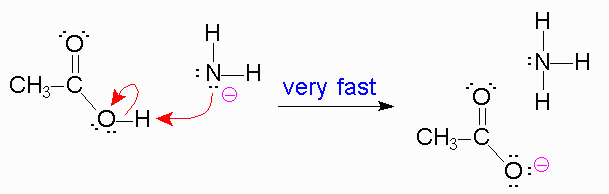
#color(blue)("Here, the NH"_2^(-) "will act as a base and pluck off the H from the carboxylic")# #color(blue)("acid - type of acid-base reaction.")# When this happens, a #"carboxylate anion"# forms and #"NH"_3#.