Question #3ca57
1 Answer
Apr 21, 2017
Because Na atom has extra electron
Explanation:
When we are talking of a
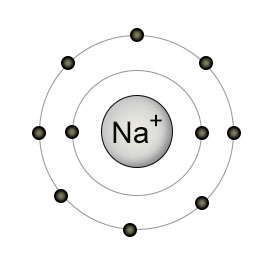
while an Na atom has
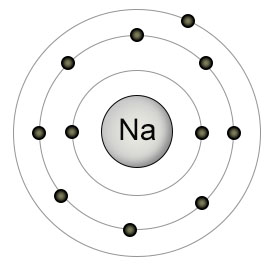
an extra electron which is the only one present in its last accessible orbital thus covering more space.If you think it as a circle.consider nucleus as the center of the circle
Whereas in Na atom

