Question #152e2
1 Answer
May 6, 2017
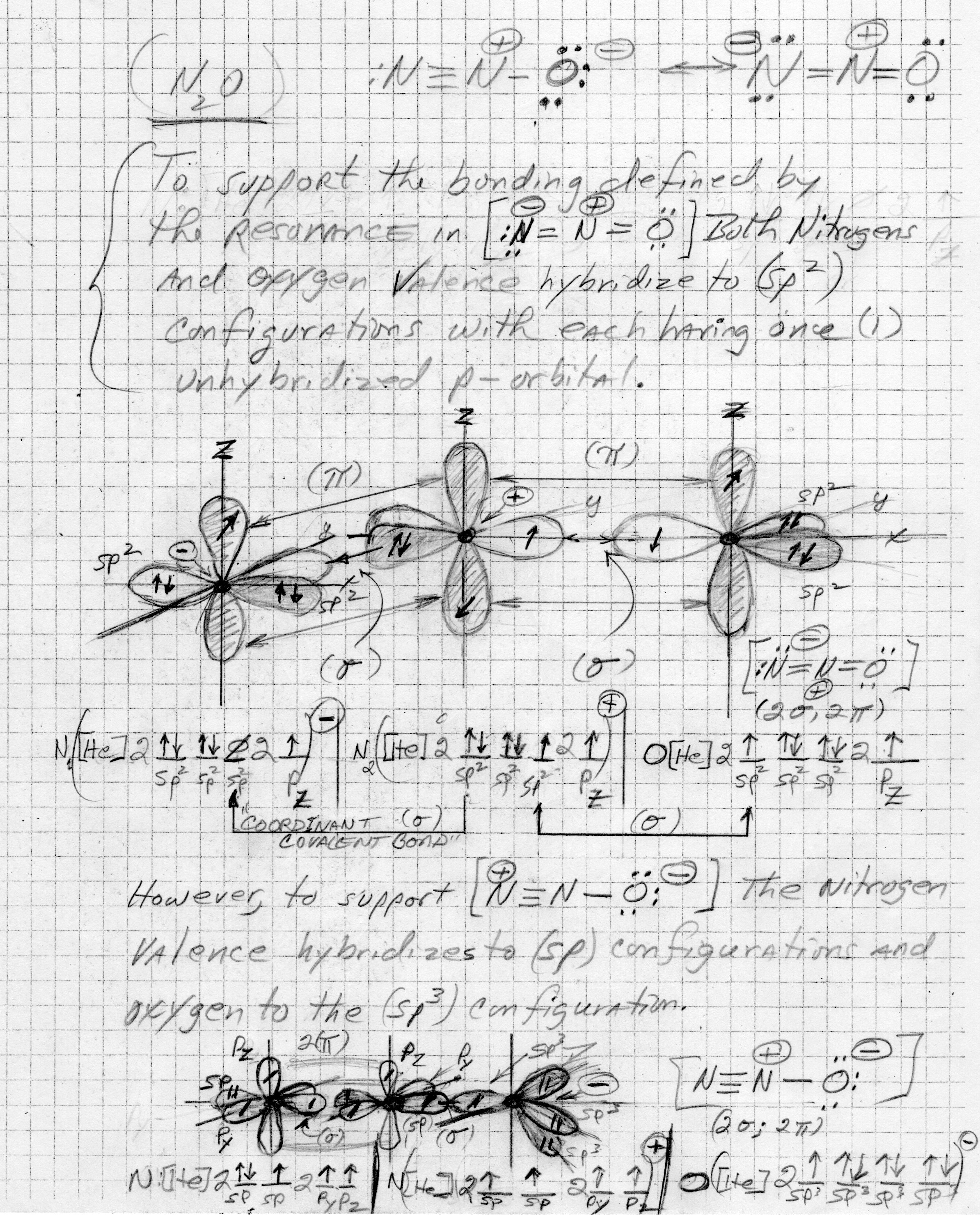
This one's tricky in that
To support the structure containing the triple bond; an
Explanation:
The graphic needed to illustrate the hybridization certainly exceeds my artistic skill set, but was unable to find a suitable graphic online.
So, with undeterred determination, here's my best effort.