We know or should know that #"electronegativity"# is defined as the ability of an atom involved in a chemical bond to polarize electron density towards itself. Electronegativity decreases across the Period, i.e. INCREASES across a row of the Periodic Table, from left to right as we face the Table, BUT DECREASES down a Group, a column of the Periodic Table. This is the result of progressively increasing nuclear charge that is shielded very poorly by INCOMPLETE valence electronic shells.
Now #c# and #d# both combine an alkali metal (an element that should be very POORLY electronegative!) with a Group 17 halogen, an atom that because of its nuclear charge and its Periodic position, should be VERY ELECTRONEGATIVE. Because potassium is LESS electronegative than sodium, #"option c."# is the required answer, because here the GREATEST difference in electronegativity is observed.
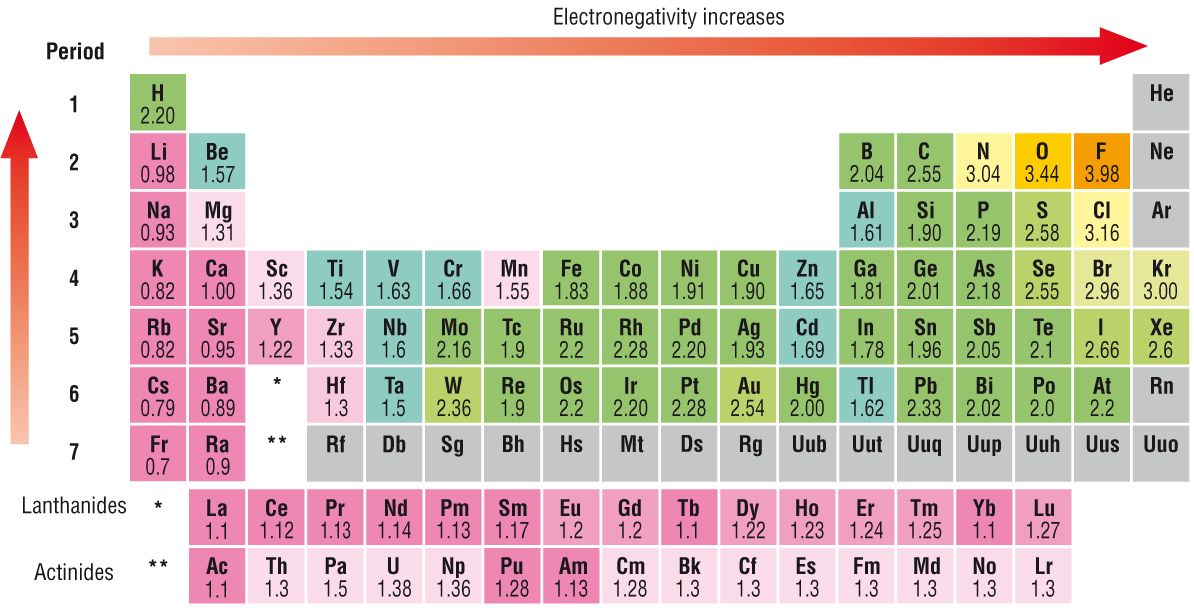
Does the diagram support what I have argued?

