What are the resonance isomers for ozone, O_3?
1 Answer
May 12, 2017
There are two major resonance contributors for ozone (
Explanation:
A stable resonance structure is one where the greatest possible number of atoms obey the octet rule, with the least possible separation of charge.
You could draw these two
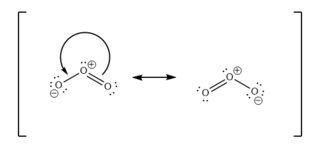
These are the two major resonance contributors (most stable).
Two minor structures can be drawn as well:

You can see that both have atoms that do not obey the octet rule and are therefore both minor contributors (and thus less stable)
If you want to know how to draw these structures yourself in the future, you could check out here.
