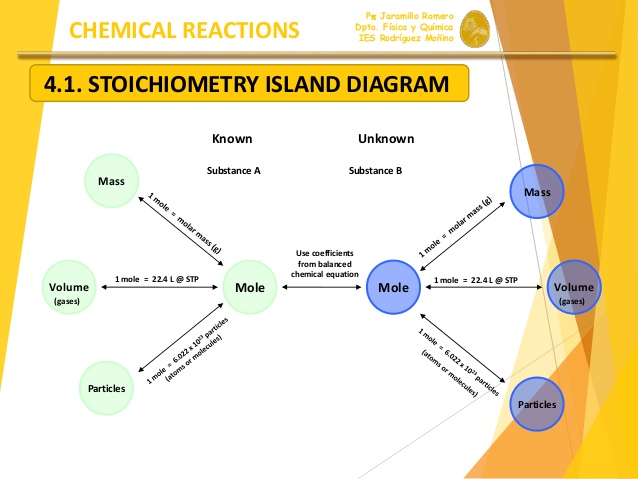
What are 3 conversion factors used in stoichiometry?
1 Answer
Molar Mass (moles
Stoichiometric Coefficients (moles
Avagadro's number (moles
Explanation:
There are a number of calculations you'll need to do in stoichiometry, but the aforementioned three are the most common.
Your molar mass is what you'll need to use to go from grams of your species to moles of your species (and vice-versa). Every element's molar mass is listed on the periodic table, and the molar mass of a compound can be found by simply adding the molar masses of all its constituent elements.
The stoichiometric coefficients tell you the molar ratio between two species in a balanced chemical equation. For example, consider the generic reaction below:
What this tells you is that every 1 mol of
Avogadro's number provides a relationship between moles and the number of atoms/molecules. It tells you that, by definition, you have
This diagram sums it up nicely:
Hope that helps :)

