How do I write the electron configuration of an oxygen molecule?
1 Answer
May 16, 2017
Here's how I do it.
Explanation:
Since
I assume that you already know how to draw a molecular orbital digram like the one below.
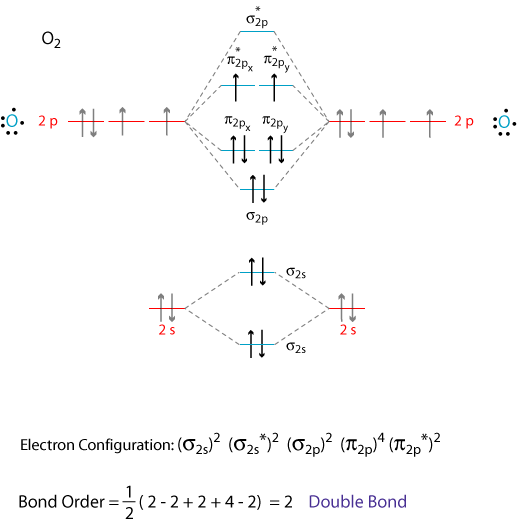
Note: The diagram above omits the
To write the electron configuration of the molecule, we just give the number of electrons in each orbital:

