Question #4f8a7
1 Answer
Well, it should...........because the acid-base reaction is REVERSIBLE.
Explanation:
The indicator is simply a LARGE, WEAK, organic acid, whose conjugate base/conjugate acid forms are readily differentiated by their different colours.
Personally, I find titrations with phenolphthalein excellent with respect to finding the stoichiometric endpoint of a reaction. A stoichiometric endpoint should be reached when you add approx.
At, or about the endpoint the colour of the solution, which is expressed by the indicator is VERY sensitive to addition of acid of base, because a few drops added cause such a large rise (or decrease) in solution
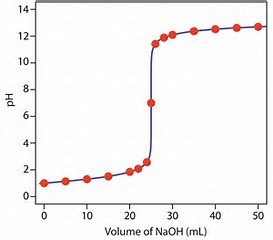
The precipitous increase in solution

