Can someone compare s, p, d, and f orbitals in terms of size, shape, and energy?
1 Answer
this is a nice question - simple to ask, difficult to answer. I'll give it a try....
Explanation:
we need to set some parameters to this question. obviously s,p,d and f all exist at different quantum levels.
but if we take quantum level n=4 (the first quantum level where all 4 sub-shells - s,p,d,f - exist) then you could begin to draw some comparisons.
For instance, you could simply the models and say that all 4 sub-shells have approximately the same diameter - that is to say that they all have similar distances from the atomic nucleus to the main area of electron density (or volume of greatest cloud probability).
But the energy of each sub-shell is vastly different - so the 4s is greatly more stable than the 4f - which is why atoms fill the 4s sub-shell before the 4f. This is of course what leads to the quantum level splitting diagram that you may be familiar with:
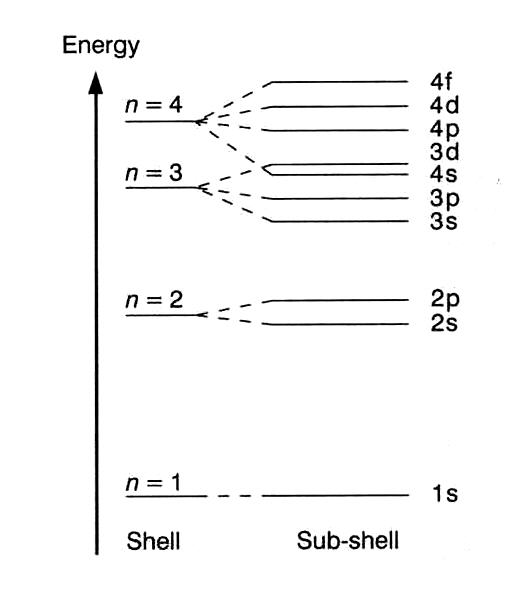 )
)
The shapes can't be compared as they are all unique. You can compare all the s orbitals - as these all have the same shape (spherical), equally all the p orbitals (dumb-bell) etc. The only difference there is size - so 1s is a small sphere, 2s is a bigger sphere, 3s even bigger sphere etc.
this might help more,
(http://hyperphysics.phy-astr.gsu.edu/hbase/Chemical/eleorb.html)

