Question #67752
1 Answer
May 29, 2017
Refer to explanation
Explanation:
A weak acid is an acid that is not able to completely dissociate into ions. For example, ethanoic acid. This is why the reaction is reversible.
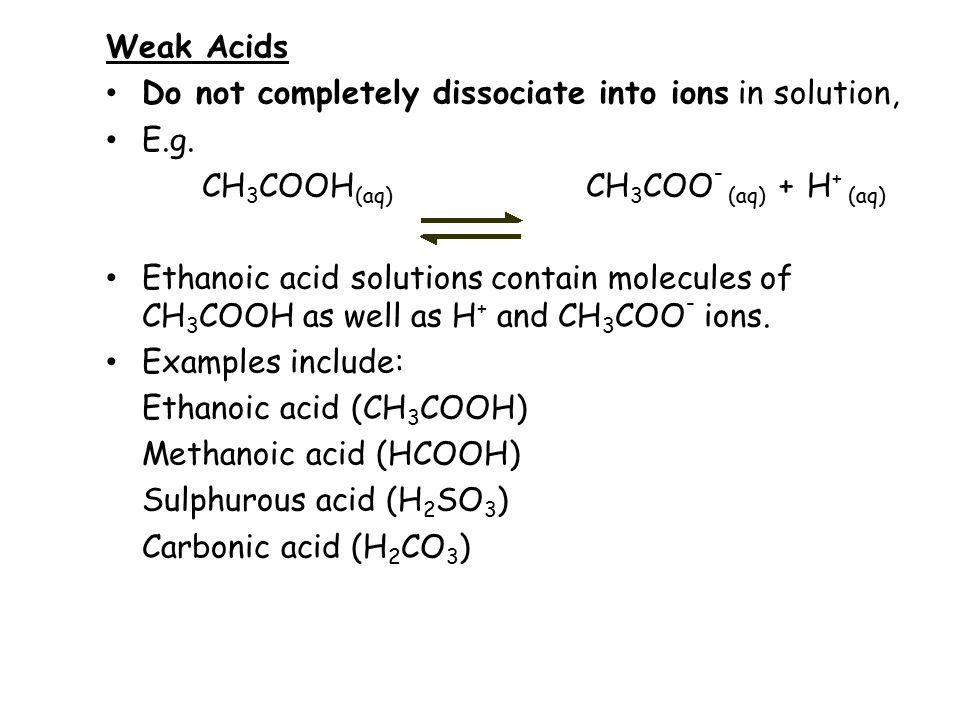
A dilute acid is just water added to the acid to reduce its concentration. The acid can be either strong or weak.

