What is the Lewis dot diagram for #H_2O#?
1 Answer
May 30, 2017
Well we have 6 valence electrons from the oxygen atom......
Explanation:
And 2 valence electrons from the hydrogen atom. And thus we have to distribute 4 electron pairs around the central oxygen atom. VESPER predicts that these 4 electron pairs will assume the shape of a tetrahedron:
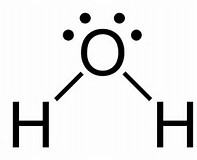
The

