Question #dde3d
1 Answer
Here's what I got.
Explanation:
You didn't provide a molarity for the acetic anhydride solution, so we cannot calculate the theoretical yield.
However, we can still go through the solution step by step and see how the molarity of the acetic anhydride solution can influence what is going on.
So, salicylic acid and acetic anhydride react in a
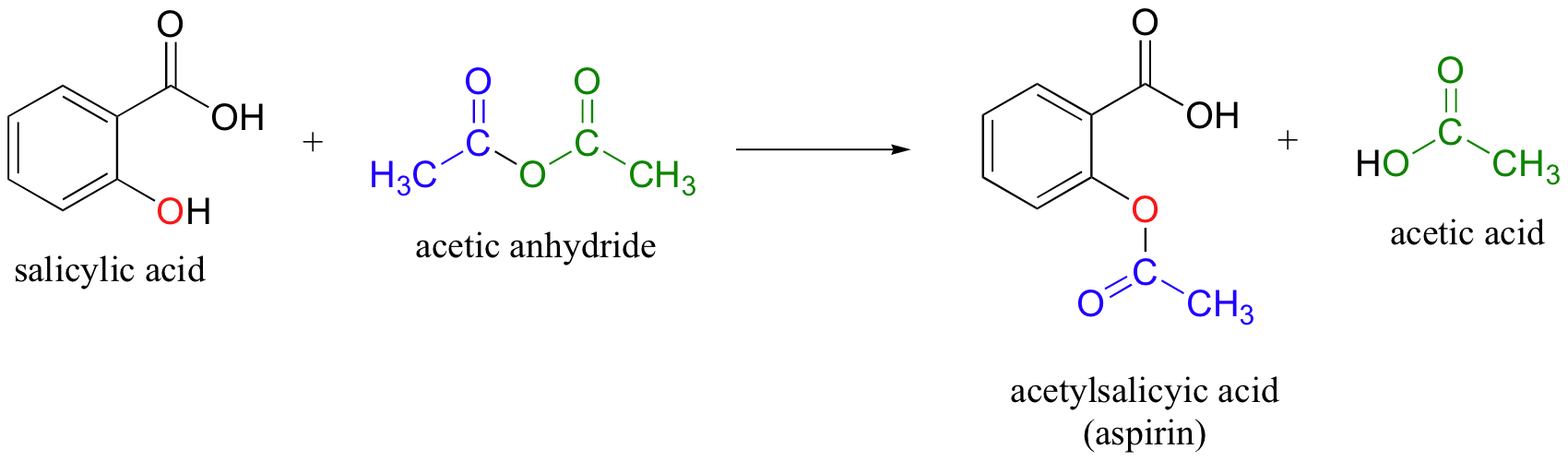
This means that for ever
Now, use the molar mass of salicylic acid to convert the mass to moles
#2.0 color(red)(cancel(color(black)("g"))) * ("1 mole C"_7"H"_6"O"_3)/(138.121color(red)(cancel(color(black)("g")))) = "0.01448 moles C"_7"H"_6"O"_3#
Now, if you take
#5.00 color(red)(cancel(color(black)("mL"))) * (xcolor(white)(.)"moles C"_4"H"_6"O"_3)/(10^3color(red)(cancel(color(black)("mL")))) = (0.005 * x)# #"moles C"_4"H"_6"O"_3#
Next, we need to make a decision about the value of
You have three choices
#color(red)(ul(color(black)(0.01448 > (0.005 * x))))# In this case, acetic anhydride is the limiting reagent, i.e. it will be completely consumed before all the moles of salicylic acid will get the chance to take part in the reaction.
The reaction will consume
#(0.005 * x)# moles of both reactants and produce#(0.005 * x)# moles of aspirin.
#color(red)(ul(color(black)(0.01448 = (0.005 * x))))# In this case, neither the salicylic acid nor the acetic anhydride act as a limiting reagent.
The reaction consumes
#0.01448# moles of both reactants and produces#0.01448# moles of aspirin.
#color(red)(ul(color(black)(0.01448 < (0.005 * x))))# In this case, salicylic acid acts as a limiting reagent, i.e. it will be completely consumed before all the moles of acetic anhydride will get the chance to react.
The reaction will consume
#0.01448# moles of both reactants and produce#0.01448# moles of aspirin.
Let's say that the salicylic acid acts as the limiting reagent since this will allow us to come up with an actual value for the theoretical yield of the reaction.
This means that the reaction produces
To convert this to grams, use the compound's molar mass
#0.01448 color(red)(cancel(color(black)("moles aspirin"))) * "180.157 g"/(1color(red)(cancel(color(black)("mole aspirin")))) = color(darkgreen)(ul(color(black)("2.6 g")))#
This represents the theoretical yield of the reaction, i.e. what you get for a reaction that has a
If the acetic anhydride acts as the limiting reagent, then the reaction produces
#(0.005 * x) color(red)(cancel(color(black)("moles aspirin"))) * "180.157 g"/(1color(red)(cancel(color(black)("mole aspirin")))) = color(darkgreen)(ul(color(black)((0.901 * x)color(white)(.)"g")))#
So, figure out the value of

