A chemist mixes a 10% saline solution with a 20% saline solution to make 500 milliliters of a 16% saline solution. How many milliliters of each solution does the chemist mix together?
2 Answers
Explanation:
To solve for two unknowns we need two equations/pieces of information. Our two unknowns are the volumes of each stock solution. Let:
For our first equation, we know the total volume is 500 mL and is the sum of x and y:
For our second equation, we do a mass balance for 500 mL of final solution.
This means that in 1 mL of solution, we have 0.16 g of NaCl.
For any solution, concentration multiplied by volume will give the mass of NaCl:
So in
So, the sum of the masses of NaCl in
Now, substitute our expression for x, (1), into (2):
Now solve for y using (1):
A different approach! Very detailed explanation given.
For the 20% constituent:
For the 10% constituent:
Explanation:
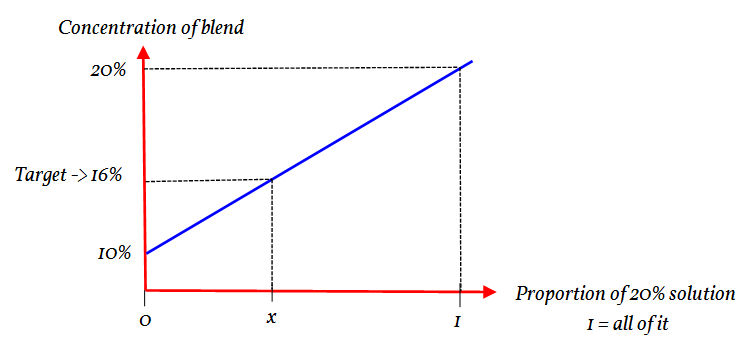
The total volume is a fixed amount. So if the proportion of the 20% concentration is known then the amount of 10% solution is:
Thus by just focusing on the 20% the amount of the 10% is indirectly linked. Thus in this approach we can (sort of) forget about the amount of 10% mix.
By varying the amount of the 20% mix the saline content of the whole changes. It is this change that we are looking at.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
 Tony B
Tony B
Let the proportion of the 20% solution be
The gradient of part is the same as the gradient of the whole.
Using ratio:
Giving
Multiply both sides by 6
......................................................................................
Thus there is:
Check:
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
For the 20% constituent:
For the 10% constituent:


