What is the ground-state electron configuration of the element Kr?
1 Answer
OR
OR
Explanation:
First: Determine the number of electrons. Krypton has a total of 36 electrons
Second: KNOW YOUR ORBITALS
Know how many electrons each orbital can hold and their order.
(Refer to the following pictures as notes)
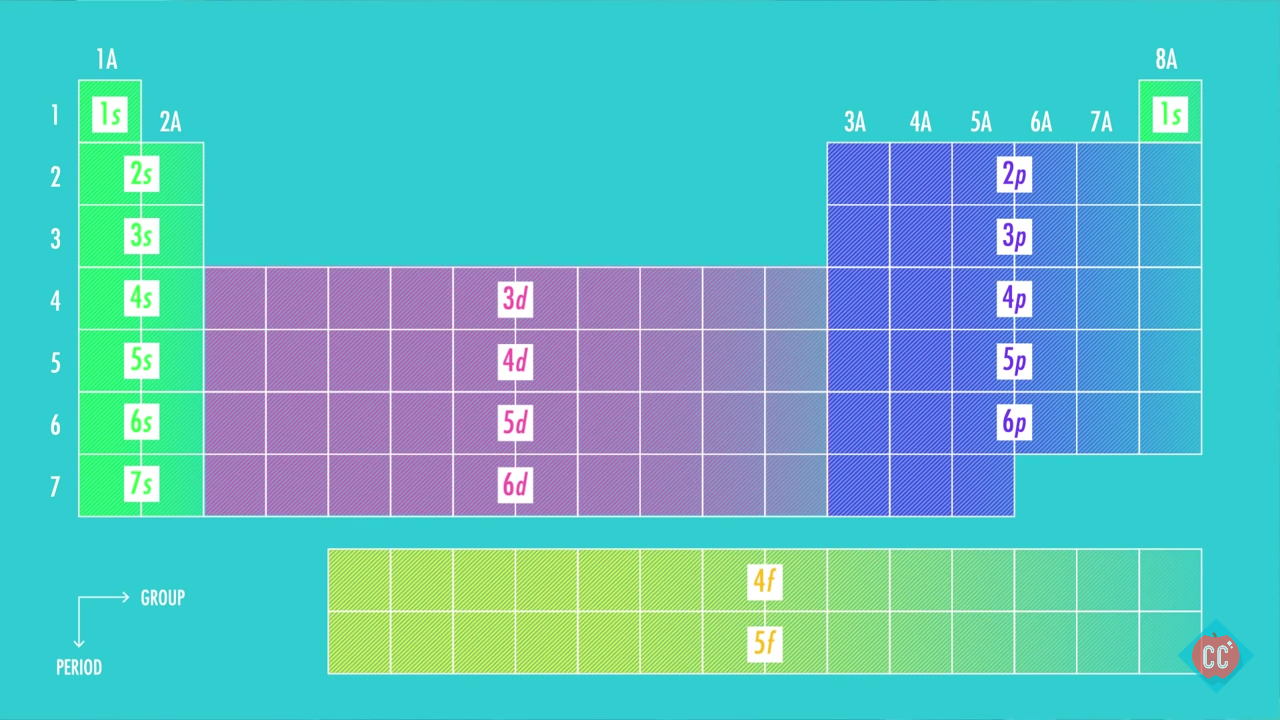
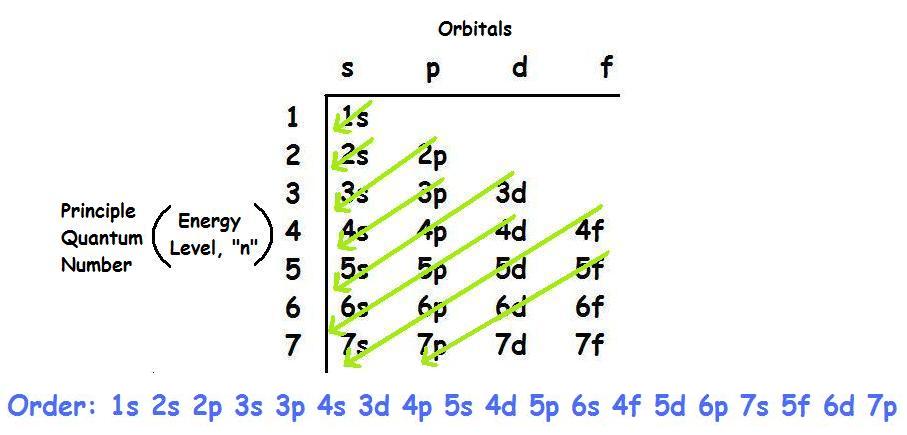
Third: Write out the electron configuration
For Krypton and most of the elements there are more the just one way (usually two) to write the electron configuration. One way is to write out the entire electron configuration by going through each orbital or we can use a shorthand notation using the noble gases as a starting point. I will go through both methods:
First Method: (Long way)
We know that Krypton has
We know that the
Second Method (Shorthand)
The key to using this method is to identify the noble gas closest to the desired element that is at a lower energy (Has a lower atomic number if I'm loosely speaking). In essence, the shorthand notation tells us the configuration by using a noble gas element as our starting point instead of starting all the way at the
Coincidently, Krypton itself is a noble gas so we could write the electron configuration as
*Notice how the configuration
All in all, the three given answers are correct ways of figuring out the ground-state electron configuration of Krypton .

