What is the average bond order in the sulfate ion?
1 Answer
Jun 19, 2017
The average bond order is 1.5.
Explanation:
Here's how I calculate the average bond order.
You draw the resonance contributors as usual.
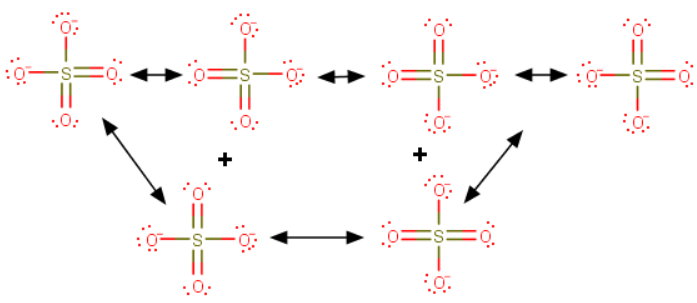
There are six equivalent contributors to the sulfate ion.
Because they are all equivalent, we need to look at only one of them.
Here's the formula:
#color(blue)(bar(ul(|color(white)(a/a) BO = "No. of bonding electron pairs"/"No. of bonded atoms"color(white)(a/a)|)))" "#
In each resonance contributor, there are six bonding electron pairs and four atoms bonded to the
#BO = 6/4 = 1.5#
The average bond order in the sulfate ion is 1.5.

