Which is a better Lewis acid, #"AlCl"_3# or #"BCl"_3#?
2 Answers
As the size of the element increases it does not want an electron and it behaves more like a Lewis Base.
Explanation:
1st Definition
Lewis acids react with Lewis bases to form a Lewis adduct. In this reaction only product is produced. Like
The Lewis base donates an unshared electron pair (lone pair)to a Lewis acid to form a Lewis adduct.
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom and is sometimes called a non-bonding pair. Lone pairs are found in the outermost electron shell of atoms(valence shell). They can be identified by using a Lewis structure.
As a result the reaction is a endothermic as more bonds are added to the product.
2nd Definition
When a base donates a lone pair to a Lewis acid the Lewis acid actually accepts it.Therefore Lewis acid is an ion or a compound that can accept an electron pair from an ion or base.
3rd Definition
In water Lewis acid form complexes(not to be confused with ions) which is a Bronsted Lowry Acid.
Due to small size of boron, the sum of its first three ionization enthalpies is very high. This prevents it from +3 ions and forces it to form only covalent compounds.
But as we move from B to Al , the sum of the first three ionization enthalpies of Al considerably decreases and is therefore able to form
However, down the group , due to poor shielding effect of intervening d and f orbitals(because of their structure), the increased effective nuclear charge holds
As a result of this only p-orbital electron may be involved in bonding. In fact in Ga , In and Tℓ, both +1 and +3 oxidation states are observed. The relative stability of +1 oxidation state progressively increases for heavier elements :
In thallium +1 oxidation state is predominant whereas the +3 oxidation state is highly oxidizing in character. The compounds in +1 oxidation state , as expected from energy considerations , are more ionic than those in +3 oxidation state.
In trivalent state, the number of electrons around the central atom in a molecule of the compounds of these elements (e.g., boron in BF3) will be only six. Such electron deficient molecules have tendency to accept a pair of electrons to accept a pair of electrons to achieve stable electronic configuration and thus, behave as Lewis acids. The tendency to behave as Lewis acid decreases with the increase in the size down the group.
Summary
As the size of the element increases it does not want an electron and it behaves more like a Lewis Base.
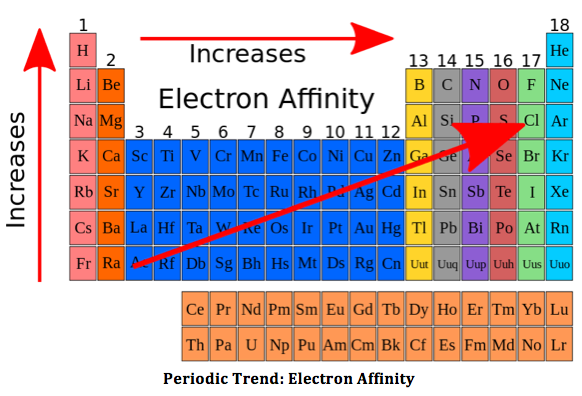
The electron affinity
4 things affecting electron affinity
-
Shielding electrons
-
Effective nuclear charge
-
distance from the nucleus
-
Noble gases will have no electron affinity as they are non-reactive
Because that the electron affinity of boron is the smallest boron will easily accept an electron. According to your question the tendency of a group 13 element to behave as a lewis acid decrease with increase in size. And that is why the aluminium has a lower electron affinity. A increase in electron affinity is seen in gallium and indium which does not apply to the trend. This is because of d orbitals which have very poor screening effect and therefore the atomic radius of gallium is less than that of aluminium. But in indium the 4d sub shell is not fully occupied which causes the atomic radius to increase .
Another way determining is using ionization energy

The greater the Ionization energy the more its ability to behave as a lewis acid.Ionization energy is the energy to remove one electron from the last shell. Therefore the more the energy required to remove a electron the more easier to add a electron.As lewis acid's work is actually to accept a electron from compound(the range of compounds affect the strength of the acid) the element should very easily accept the electron in order to become a lewis acid.
The tendency of a group 13 element to behave as a lewis acid in decreasing order.
And this is also the order of increasing atomic radii
In boron the atomic radius is the smallest and in Thallium the atomic radius is maximum
Lewis acidity within the realm of molecular orbital theory is directly proportional to the ability of the lowest-occupied molecular orbital (LUMO) to accept electron density from a Lewis base's highest-occupied molecular orbital (HOMO).
That is, the better the LUMO can accept electron density, the stronger the Lewis acid.
If we take
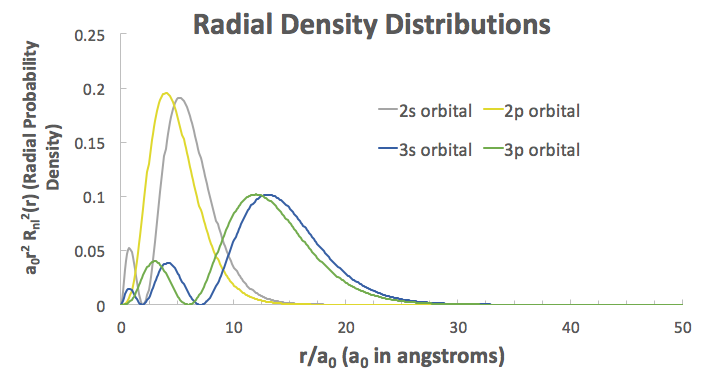
That raises the energy of the antibonding MOs and lowers the energy of the bonding MOs.
This is because the relative increase in size of these orbitals promotes greater overlap and thus greater interaction because the radial extents of the
Here are examples from the hydrogen atomic wave functions (relative comparison only):
Among these antibonding MOs is the LUMO (which primarily belongs to the central atom), labeled the
[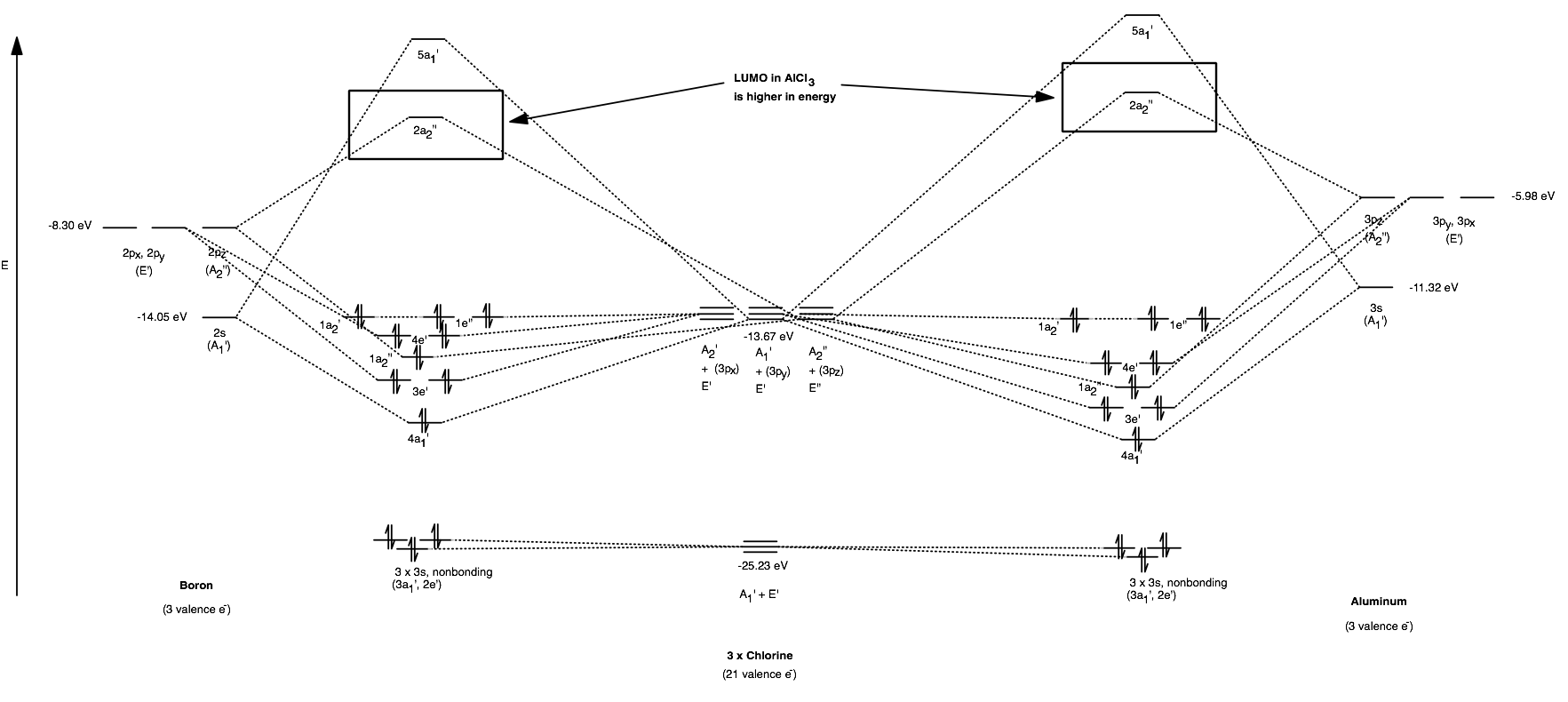
(You can click on the image to zoom in. Energy data was from Appendix B.9. A reference MO diagram [Miessler et al.] for relative energy comparisons can be found for
The LUMO is what accepts electrons when
Assuming the same interacting Lewis base (such as
(I'm not going to say it's a general trend, but it proves true for these two central atoms.)


