Can someone make this clear for me by explaining, why 4s comes before 3d, when writing the electronic configuration of elements? For example, chromium, its electron configuration is, 1s2 2s2 2p6 3s2 3p6 4s1 3d5. Why 4s isn't after 3d?
2 Answers
Because before scandium, the
#[Ne]3s^2 3p^6 4s^2#
At scandium and past, the
You can read more about that here.
CONSIDERATIONS REGARDING ORBITAL ENERGIES
It's just become a bad "habit" to write
#bb([Ar]3d^? 4s^?)#
This indicates that the
ORBITAL POTENTIAL ENERGIES
See how the
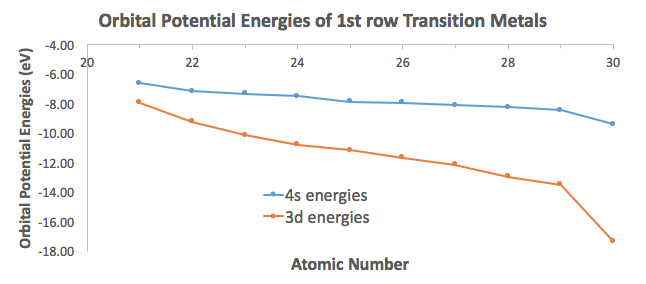
And you can further see how the Aufbau principle fails for the heavier transition metals, in that the
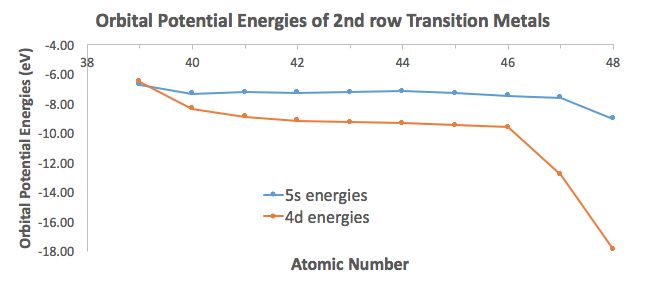
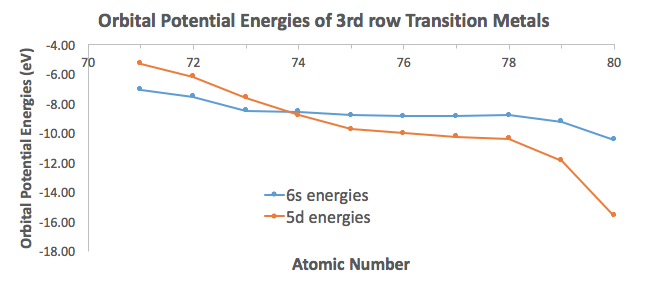
The electron configuration of chromium usually is written with
Explanation:
Every single resource I have checked had
As it turns out, for the first row of transition metals, the 4s sublevel has higher energy than the 3d sublevel, which is contrary to what we would expect from an Aufbau diagram. So the electron configuration for chromium is written in order of increasing energy, with the 4s sublevel having the highest energy and written at the end.
Thank you to Truong-Son N. :)


