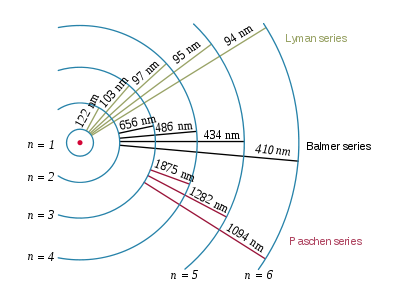
An excited hydrogen atom with an electron in the n = 5 state emits light having a frequency of 6.90 xx 10^14 s^-1. What is the principal quantum level for the final state in this electronic transition?
1 Answer
Explanation:
The first thing that you need to do here is to use the frequency of the emitted photon to calculate its wavelength.
As you know, frequency and wavelength have an inverse relationship described by the equation
color(blue)(ul(color(black)(nu * lamda = c)))
Here
nu is the frequency of the photonc is the speed of light in a vacuum, usually given as3 * 10^8 "m s"^(-1)
Plug in your value to find
lamda = (3 * 10^8color(white)(.)"m" color(red)(cancel(color(black)("s"^(-1)))))/(6.90 * 10^(14)color(red)(cancel(color(black)("s"^(-1))))) = 4.348 * 10^(-7) "m"
Now, the relationship between the wavelength of the emitted photon and the principal quantum numbers of the orbitals from which and to which the transition is being made is given by the Rydberg equation
1/(lamda_"e") = R * (1/n_1^2 - 1/n_2^2)
Here
lamda_"e" is the wavelength of the emitted photon (in a vacuum)R is the Rydberg constant, equal to1.097 * 10^(7) "m"^(-1) n_1 represents the principal quantum number of the orbital that is lower in energyn_2 represents the principal quantum number of the orbital that is higher in energy
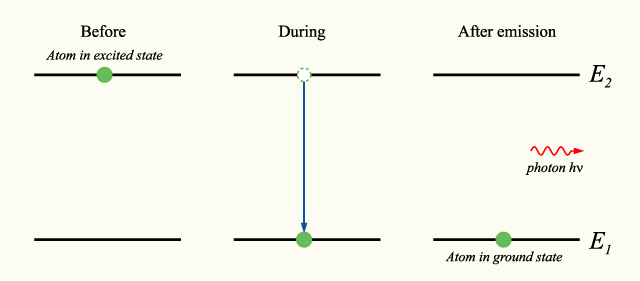
Now, you know that your electron emits an electron, so right from the start, you know that the principal quantum number of the orbital that is higher in energy will be
In other words, the principal quantum number of the final orbital in this transition must be
So, rearrange the Rydberg equation to isolate
1/(lamda_ "e") = R/n_1^2 - R/n_2^2
R/n_1^2 = 1/(lamda_ "e") + R/n_2^2
R/n_1^2 = (n_2^2 + lamda_ "e" * R)/(lamda_ "e" * n_2^2) implies n_1 = sqrt((R * lamda_ "e" * n_2^2)/(n_2^2 + lamda_ "e" * R))
Plug in your values to find
n_1 = sqrt(( 1.097 * color(blue)(cancel(color(black)(10^7))) color(red)(cancel(color(black)("m"^(-1)))) * 4.348 * color(blue)(cancel(color(black)(10^(-7))))color(red)(cancel(color(black)("m"))) * 5^2)/(5^2 + 4.348 * color(blue)(cancel(color(black)(10^(-7))))color(red)(cancel(color(black)("m"))) * 1.097 * color(blue)(cancel(color(black)(10^7)))color(red)(cancel(color(black)("m"^(-1))))))
n_1 = 2.001384 ~~ 2
Therefore, you can say that your electron is undergoing a