Question #b0d55
1 Answer
Here's what I got.
Explanation:
For starters, you need to have a balanced chemical equation to go by here.
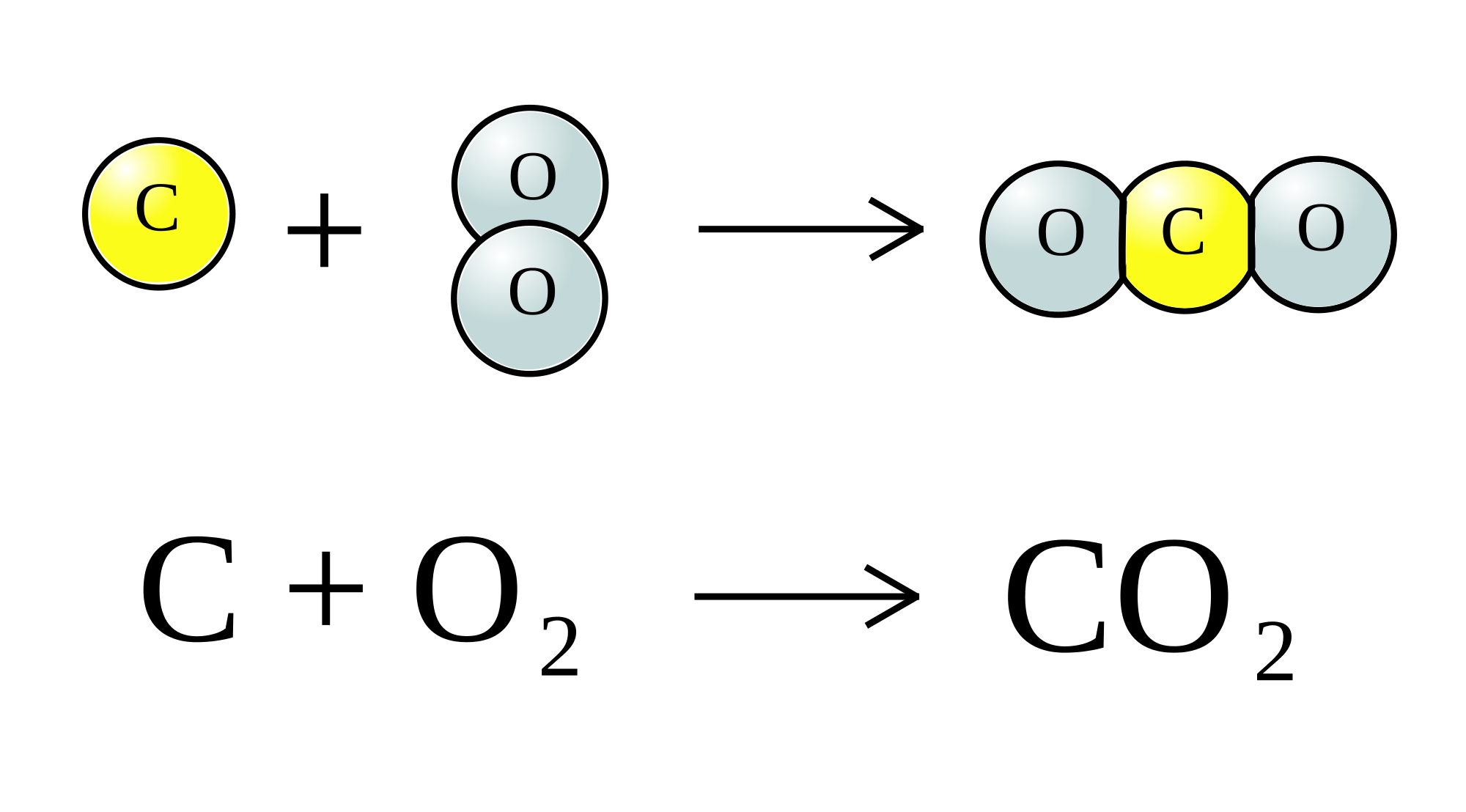
As you can see, carbon will react with oxygen gas in a
This means that the number of moles of carbon that take part in the reaction will be equal to the number of moles of carbon dioxide produced by the reaction.
Now, you know that the reaction produced
#50 color(red)(cancel(color(black)("g"))) * "1 mole CO"_2/(44.01color(red)(cancel(color(black)("g")))) = "1.136 moles CO"_2#
You can thus say that the reaction produced
To convert this to grams, use the element's molar mass
#1.136 color(red)(cancel(color(black)("moles C"))) * "12.01 g"/(1color(red)(cancel(color(black)("mole C")))) = color(darkgreen)(ul(color(black)("14 g")))#
I'll leave the answer rounded to two sig figs, but keep in mind that you only have one significant figure for the mass of carbon dioxide.

