Why is the ketone more stable than its tautomer enol?
1 Answer
Well, sometimes it's not..........but enol formation in
Explanation:
You know that ketones, and aldehydes, undergo some degree of
Sometimes, this equilibrium can be driven towards the RHS, the enol side by treating the starting material with a strong base to form the
In general for
This so-called
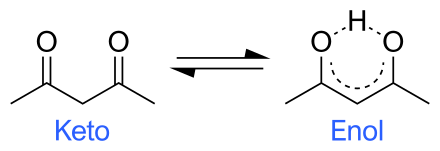
And in the diagram enol formation is facilitated by the formation of a stable, heterocylic six-membered ring that is stabilized by hydrogen bonding.........

