#"AlF"_3#? It doesn't have to. Neither do #"AlCl"_3#, #"BF"_3#, etc.
Aluminum comes in with #bb3# valence electrons, and each fluorine comes in with #bb7# valence electrons. That gives
#3 + 3 xx 7 = bb24# total valence electrons to distribute.
Each #"F"# atom often holds three lone pairs, accounting for #(3 xx 2) xx 3 = 18# of these #24#. So, the remaining #6# can go into making three single bonds, each bond using #2# valence electrons.
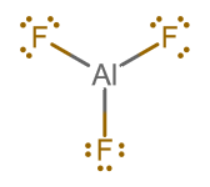
And the central atom, #"Al"#, has an empty #3p_z# orbital. That is mainly why #"AlF"_3# is a Lewis acid.

