Calcium oxide is a base compound which can react with acids such as nitric acid, HNO3. A student mixed 1.5g of CaO with 2.5M HNO3. The chemical equation for this reaction is: CaO + HNO3 = Ca(NO3)2 + H2O. Solve the following questions?
1 Answer
Here's what I get.
Explanation:
a) Type of reaction
The chemical equation is
In one sense, this is a double displacement reaction, because
In another sense, this is an acid-base neutralization reaction, because the base (calcium oxide) is reacting with the acid (nitric acid) to produce a salt (calcium nitrate) and water.
b) Adding the base to the acid
(i) Using a a pH meter
At the start, the
As you add the solid
The pH will gradually increase, perhaps to pH 2.
As you get closer to complete neutralization, the pH will increase rapidly to pH and then to some higher value, perhaps pH 12.
As you continue adding
If you were adding the
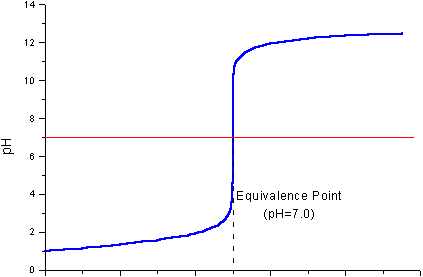
(Adapted from Chemistry Stack Exchange)
(ii) Using Universal Indicator
One type of Universal Indicator has the following colours.

Thus, you would probably start with an orange solution.
As you add

