What is the electron configuration for the f block?
2 Answers
The f block has the lower level filled, then for valance electrons has 2 s electrons 1 d electron and then up to 14 f electrons filling the 7 f orbitals.
I finally feel confident enough to post a table of the configurations, along with some detailed rationale for why the configurations are so riddled with 'Aufbau exceptions'.
For reference, the energy scales I will be using are small. For perspective, you can compare the numbers with the first ionization energy of
The following graphs are from page
DISCLAIMER: LONG ANSWER!
LANTHANIDES
The order by atomic number is down the first column, and then down the second column. In
color(white)([(color(red)(La),(color(red)([Xe] 6s^2 5d^1)),color(black)(Tb),(color(black)([Xe] 6s^2 4f^9))),(color(red)(Ce),(color(red)([Xe] 6s^2 4f^1 5d^1)),color(black)(Dy),(color(black)([Xe] 6s^2 4f^10))),(color(black)(Pr),(color(black)([Xe] 6s^2 4f^3)),color(black)(Ho),(color(black)([Xe] 6s^2 4f^11))),(color(black)(Nd),(color(black)([Xe] 6s^2 4f^4)),color(black)(Er),(color(black)([Xe] 6s^2 4f^12))),(color(black)(Pm),(color(black)([Xe] 6s^2 4f^5)),color(black)(Tm),(color(black)([Xe] 6s^2 4f^13))),(color(black)(Sm),(color(black)([Xe] 6s^2 4f^6)),color(black)(Yb),(color(black)([Xe] 6s^2 4f^14))),(color(black)(Eu),(color(black)([Xe] 6s^2 4f^7)),color(black)(Lu),(color(black)([Xe] 6s^2 4f^14 5d^1))),(color(red)(Gd),(color(red)([Xe] 6s^2 4f^7 5d^1)),"","")])
The exceptions can be explained by looking at how the energies of the
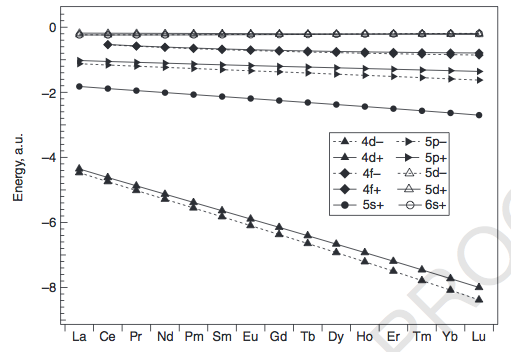
We can see that the
The radii of the
The exceptions occur mainly for the earlier lanthanides (
-
The radial compactness of the
4f orbitals makes it more favorable to fill the6s and5d first forLa andCe , to minimize electron repulsion. -
For
Gd , the repulsion that would be generated from pairing a4f electron would be enough to promote it to a5d orbital (about0.6 E_h away, or about"16 eV" ), soGd takes on af^7 d^1 configuration instead off^8 d^0 .
ACTINIDES
The order by atomic number is down the first column, and then down the second column. In
color(white)([(color(red)(Ac),(color(red)([Rn] 7s^2 6d^1)),color(black)(Bk),(color(black)([Rn] 7s^2 5f^9))),(color(red)(Th),(color(red)([Rn] 7s^2 6d^2)),color(black)(Cf),(color(black)([Rn] 7s^2 5f^10))),(color(red)(Pa),(color(red)([Rn] 7s^2 5f^2 6d^1)),color(black)(Es),(color(black)([Rn] 7s^2 5f^11))),(color(red)(U),(color(red)([Rn] 7s^2 5f^3 6d^1)),color(black)(Fm),(color(black)([Rn] 7s^2 5f^12))),(color(red)(Np),(color(red)([Rn] 7s^2 5f^4 6d^1)),color(black)(Md),(color(black)([Rn] 7s^2 5f^13))),(color(black)(Pu),(color(black)([Rn] 7s^2 5f^6)),color(black)(No),(color(black)([Rn] 7s^2 5f^14))),(color(black)(Am),(color(black)([Rn] 7s^2 5f^7)),color(black)(Lr),(color(black)([Rn] 7s^2 5f^14 6d^1))),(color(red)(Cm),(color(red)([Rn] 7s^2 5f^7 6d^1)),"","")])
We can again examine the energies (pg. 199):
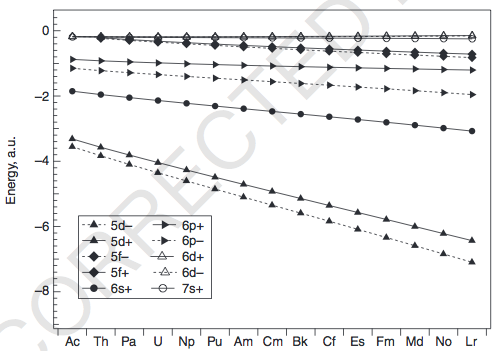
The energies of the
That makes the energetic degeneracies of the
As before, the exceptions occur mainly for the earlier actinides (
- For
Ac-Th , since the5f 's and6d 's are very similar in energy, it is possible for6d occupation instead of the5f . I believe it is because the5f orbitals are barely bigger than the6p ,6s , and5d orbitals forTh that the5f is about as core-like as them and thus not as accessible to fill... but this is difficult to explain.
You can see the radial extents here (pg. 202):
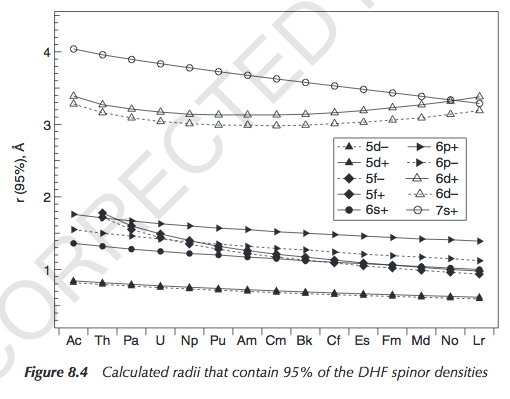
"Spinor" just means an electronic quantum state (in the Pauli Exclusion sense) with a specific spin (up/down). DHF stands for Dirac-Hartree-Fock.
-
For
Pa - Np , whose6d-5f gap is even smaller than the5d-4f gap of the lanthanides (but bigger than forAc-Th ), I believe that the repulsions generated from adding a second electron into the6d orbitals (even without pairing) are still enough that since the5f orbitals are lower in energy, it is preferable to proceed by filling them instead. -
And again for
Cm , similar toGd , the electron repulsion that occurs with pairing an5f electron would be enough to promote it to a6d orbital (about0.15 E_h away, or about"4 eV" ).

