What are the electron configurations of carbon, hydrogen, and oxygen atoms?
1 Answer
Jul 23, 2017
Explanation:
Why are they such?
Since each atom has various electron shells to fill, the 1s, 2s and 2p subshells of each atom contain up to two electrons per orbital depending on the amount of protons in the nucleus (constrained by conservation of charge).
For instance, hydrogen has the lowest energy shell, 1s, containing one electron, since a neutral atom of hydrogen has 1 proton and the electron balances the
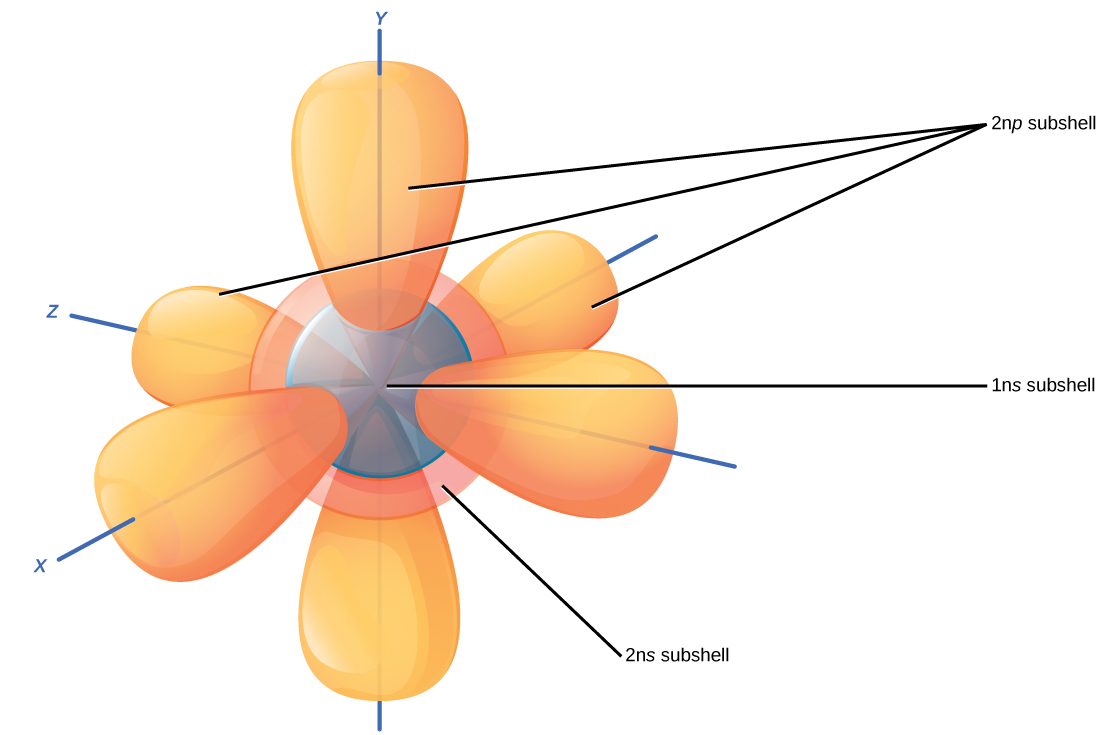
Here's an illustration of what I'm trying to simply explain! Each subshell contains up to two electrons per orbital. Hence, in hydrogen only the 1s orbital contains the one electron, while the others are empty until the atom acts or is acted upon.


