What is the bond order of #"H"_2^+#?
1 Answer
Aug 7, 2017
Well, we begin from the
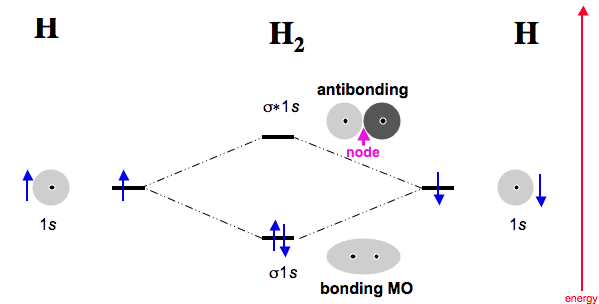
The
- one
#sigma_(1s)# bonding MO. - one
#sigma_(1s)^"*"# antibonding MO.
Changes to bond order (corresponding to bond strength) can be summarized as follows:
- Putting electrons into a bonding MO increases the bond order by
#1/2# per electron (strengthening the bond). - Putting electrons into an antibonding MO decreases the bond order by
#1/2# per electron (weakening the bond).
And vice versa for taking out electrons.
Therefore, the
Thus, the bond order is

