Which one is the electron configuration of #"Fe"^(+)#? #[Ar] 3d^5 4s^1#, or #[Ar]3d^6#?
1 Answer
Neither of them are correct! The correct electron configuration is
Consider the following diagram...
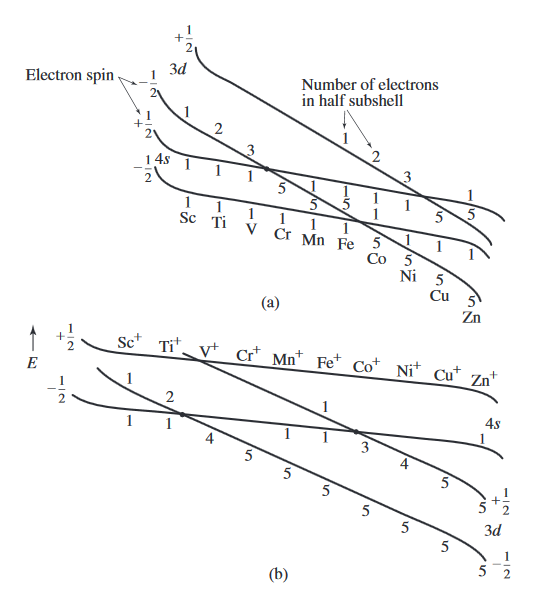
By the convention given in the diagram, the first electron in an orbital is taken to have spin down,
#overbrace(E_(3d(m_s = -1//2)))^("5 electrons") < overbrace(E_(4s(m_s = -1//2)))^"1 electron" < overbrace(E_(3d(m_s = +1//2)))^"1 electron" < overbrace(E_(4s(m_s = +1//2)))^"1 electron"#
This means for
That establishes the electron configuration
In diagram
#overbrace(E_(4s(m_s = -1//2)))^"1 electron" < overbrace(E_(3d(m_s = -1//2)))^("5 electrons") < overbrace(E_(4s(m_s = +1//2)))^"1 electron" < overbrace(E_(3d(m_s = +1//2)))^"1 electron"#
This then agrees with experiment, that the electron configuration of
#color(blue)ul([Ar]3d^6 4s^2)#
Now you may wonder,
"why is the
#4s# ionized instead of the#3d# first, even though in#Fe# , the sixth#3d# electron is highest in energy?"
The radial extent of the
The
So, the

