How does ionic radius differ from atomic radius?
1 Answer
Aug 28, 2017
Well, reasonably elemental anions should have a radius GREATER than the atomic radius.........Do you agree? Why
Explanation:
And elemental cations should have a SMALLER radius than the atomic radius. As chemists, as physical scientists we should examine the data......
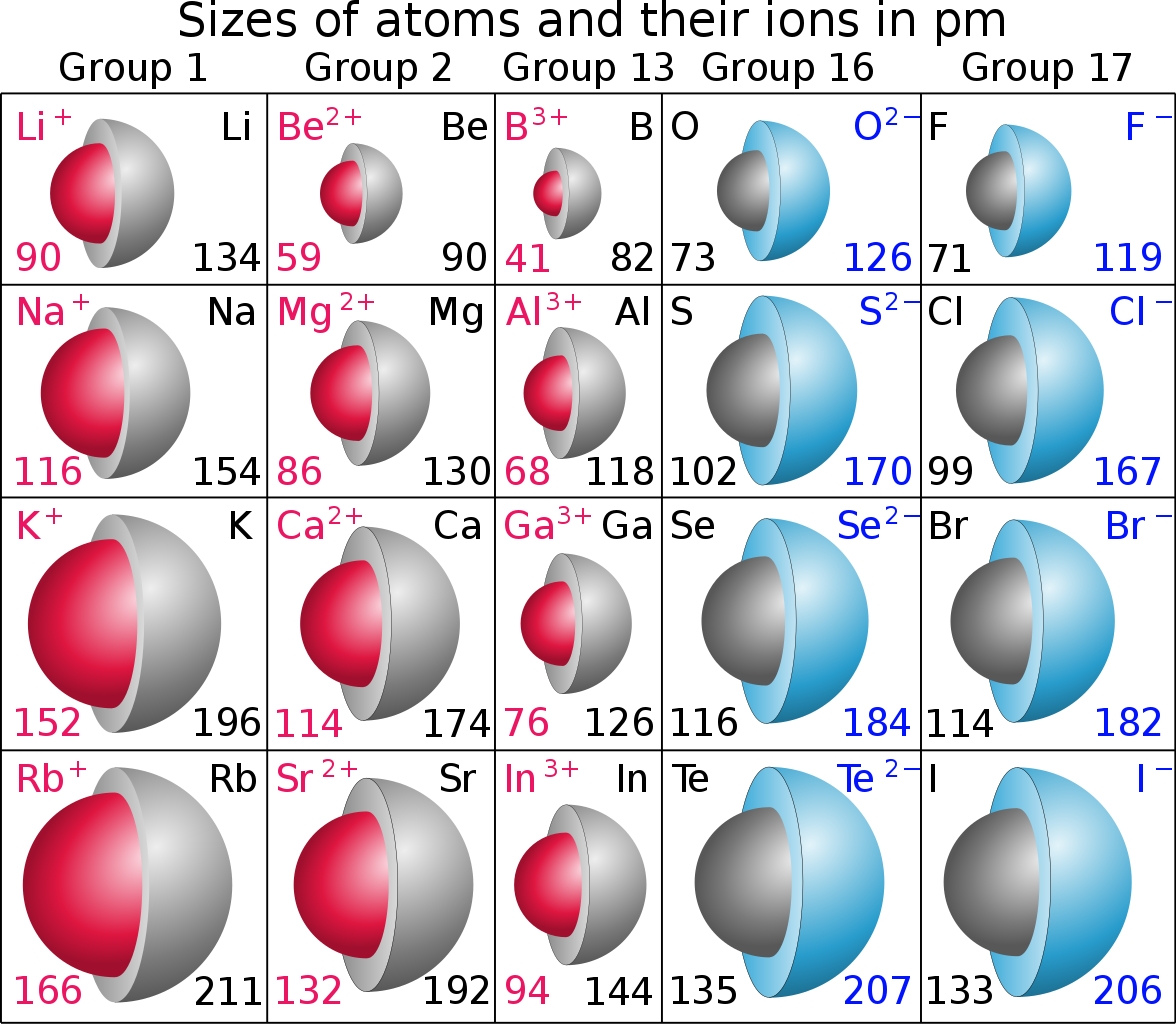
Are the data here consistent with what I have argued? The units are quoted in

